The article presents the corrosion behavior of high entropy alloy block materials and coatings in ambient, high temperatures and some special media, discusses the influence of alloying elements, heat treatment, environmental factors and manufacturing processes on the corrosion resistance of high entropy alloys, and briefly reviews the problems facing research on corrosion resistance of high entropy alloys.
High entropy alloys have gained popularity since the introduction of the high entropy alloy design concept by Yeh et al. has attracted great attention from many scientists. High entropy alloys contain five or more elements, with the content of each element ranging from 5% to 35%. As a new type of alloy with multiple main elements, high entropy alloy breaks the concept of a single main element of traditional alloy and creates a new way of thinking about alloy design.
According to traditional alloy theory, the more main elements there are, the easier it is to form complex phases such as intermetallic compounds, resulting in a significant decline in alloy performance.
Although a high-entropy alloy contains several major elements, it can form a single solid solution structure. Therefore, high entropy alloys have many unique properties that differ from traditional alloys. Particularly in terms of mechanical behavior, they present excellent properties such as high strength, hardness, wear resistance and resistance to high temperatures.
High entropy alloys are currently a new focus of research.
The developed and investigated alloy systems can be divided into two categories:
One category is Al-based alloy systems and cycle IV elements Fe, Co, Ni, Cr, Cu, Mn, Ti, such as: B. FeCoNiCrMn, AlCoCrFeNi etc.; the other category is high entropy refractory alloys based on metallic refractory elements Mo, Ti, V, Nb, Hf, Ta, W etc.
For example, TaNbHfZrTi, NbMoTaW, etc. Research into the mechanical properties and toughness of these two types of alloys has produced many high-quality results.
On the other hand, the corrosion resistance of high entropy alloys still needs to be explored. As a new type of structural material, the corrosion resistance of high entropy alloys needs to receive sufficient attention.
Researchers mainly prepare high-entropic alloy bulk materials by vacuum melting or powder metallurgy methods, and high-entropic alloy coatings on the surface of base materials by laser cladding or magnetron sputtering.
The corrosion test environment includes room temperature or high temperatures; NaCl solution, H 2 THEN 4 solution or some special media. The study of the corrosion behavior of high entropy alloys mainly focuses on the influencing factors, corrosion properties and corrosion mechanism.
1. Corrosion of high entropy alloys in media at room temperature
1.1 Influence of alloying elements on the corrosion resistance of high entropy alloys
1.1.1 Al
In the AlCoCrFeNiTi alloy, Al helps improve corrosion resistance. Qiu et al. investigated the corrosion resistance of Al Internship.
The polarization curves showed that the corrosion resistance of the alloy increased at x = 0.6 and x = 0.9 and the surface oxides were dominated by Al 2 Ó 3 with the presence of Cr 2 Ó 3 Fé 2 Ó 3 Com 3 Ó 4 and NiO. Lu et al. investigated the corrosion resistance of Al X CoCrFeNiTi 0.5 (x=0.0.5,1.0) Alloy in 0.5MH 2 SO 4 .
The results show that increasing the Al content suppresses the pitting tendency of the alloy. The corrosion resistance of the alloy is better than that of 304 stainless steel when x = 0.5 and x = 1, as shown in Fig.
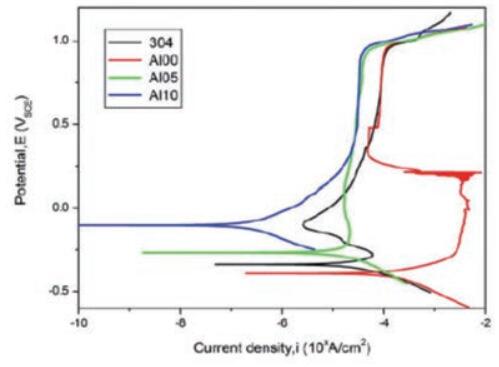
Fig. 1 Polarization curve of AlxCoCrFeNiTi 0.5 Alloy in 0.5 MH 2 THEN 4 Solution
Furthermore, Ryu and Hong et al. pitting corrosion of Al X CrFeMoV alloys (x=0,0,2,0,6,1) in 3.5% NaCl solution.
The addition of Al reduced the current density in the passivation zone of the alloys and did not reduce the pitting potential. The addition of Al had no significant adverse effects on the corrosion resistance of the alloys; however, for some high entropy alloys, the addition of Al reduces their corrosion resistance.
In Ti-free AlCoCrFeNi alloys, Liaw and Yang et al. showed that the corrosion resistance of alloys in a 3.5% NaCl solution decreased with increasing Al content.
The reason is that with increasing Al content, the microstructure of the alloy transforms from a single solid solution to a multiphase solution with an increase in the volume fraction of Al-rich phases, resulting in a decrease in the homogeneity and protection of the passivation film. . Shih et al. investigated the corrosion behavior of Al X CrFe 1.5 MnNi 0.5 alloys.
They found that Al-free alloys have wider passivation ranges than alloys containing Al at 0.5 MH. 2 THEN 4 and a lower passivation current density. Small passivation current density. In a 1MNaCl solution, the corrosion potential of an aluminum-containing alloy is significantly lower than that of an aluminum-free CrFe. 1.5 MnNi 0.5 Alloy.
1.1.2Ti
Recent studies have shown that the element titanium facilitates the passivation of high-entropy alloys. Liu et al. produced AlCoCrFeNiTi X (x=0,0,2,0,4,0,6,0,8,1,0) high entropy alloy coatings on AISI 1045 steel by laser melting. The best corrosion resistance of AlCoCrFeNiTi 1.0 The coating was obtained in 3.5% NaCl solution. The addition of Ti facilitated the passivation behavior of the coating in the NaCl solution.
The compositions of the passivation film were Al 2 Ó 3 TiO 2 Ti 2 Ó 3 Cr 2 Ó 3 and Cr.
Corrosion behavior of AlCrFeNiMo 0.5 Ti X Alloy in 3.5% NaCl solution was investigated.
The alloy consists of a BCC phase similar to FeCr and an intermetallic phase similar to NiAl. The Ti element is enriched in the NiAl-like phase, and the corrosion reaction mainly occurs in the NiAl-like phase. As the Ti content increases, the passivation film on the alloy surface has a stronger protective effect.
Al 2 CoCrCuFeNiTi The corrosion current density of high entropy alloy coatings at 0.5 mol/LH 2 THEN 4 Solution was significantly reduced compared to that of Q235 steel. In addition to Ti 1.0 Alloy coating, all other alloy layers showed passivation phenomenon.
1.1.3 months
Wang et al. investigated the effect of Mo element on the corrosion resistance of Ni 2 CrFeMo X high entropy alloy in 3.5% NaCl solution. The corrosion resistance of molten Ni 2 CrFeMo the best corrosion resistance.
Furthermore, the content of the Mo element increases, the alloy precipitates σ – Phase that causes corrosion of the galvanic coupling. On the contrary, the corrosion resistance of the alloy decreases, as shown by the electrochemical impedance spectra in Fig. 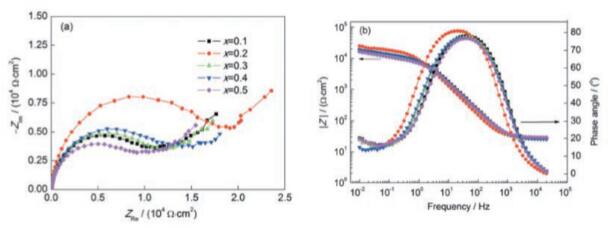
Figure 2 EIS of Ni2CrFeMo X High Entropy Alloy in 3.5% NaCl Solution
Investigation of (CoCrFeNi) 100x Mo X Alloy shows that its corrosion resistance in 3.5% NaCl solution varies with Mo content in a similar way to Ni 2 CrFeMo X Alloy. The polarization curves of CoCrFeNiMo 1 and CoCrFeNiMo 2
Alloys show that increasing Mo content improves the corrosion resistance of alloys. For CoCrFeNiMo 3 Alloy, the S phase consisting of Cr and Mo precipitates at grain boundaries, which reduces corrosion resistance. Furthermore, the polarization curves of the alloys are at 0.5MH 2 THEN 4
The solution was characterized by clear passivation. The corrosion current density gradually decreased with increasing Mo content.
Chou et al. measured the kinetic potential polarization curves of Co 1.5 CrFeNi 1.5 Ti 0.5 Mo than that of alloys containing Mo.
Furthermore, cyclic polarization tests and scanning electron microscopy observations in NaCl solution showed that Mo-containing alloys were not susceptible to pitting corrosion.
Zhang et al. investigated the corrosion resistance of TiZr 0.5 NbCr 0.5 v The alloy consists of BCC matrix phase and Cr 2 Zr phase, and a clear dendritic structure is observed. The CCC phase is mainly concentrated in the dendrites, and the ordered Cr 2 Zr phase lies between the dendrites. For Zr 0.5 NbCr 0.5 Mo Alloy: The corrosion resistance of dendrites enriched with Mo element is low.
1.1.4 Cu
An increase in the content of the element Cu generally weakens the corrosion resistance of high entropy alloys. Liu et al. investigated the corrosion behavior of CuCrFeNiMn alloy in 1M H 2 SO 4 Solution. They found that CuCr 2 Fe 2 No 2 Mn 2 Alloys with low Cu content and low degree of element segregation exhibit better corrosion resistance. In contrast to 2 CrFe 2 NiMn 2 Alloys with high Cu content and element segregation showed low corrosion resistance. Wu et al. found that the local corrosion tendency of FeCoNiCrCu X Alloys in 3.5% NaCl solution increased in size with increasing Cu content. This is due to the formation of a galvanic corrosion cell with a significant potential difference between the Cu-rich interdendritic phase and the Cu-poor dendritic phase of the alloy.
1.1.5Ko
Al 2 CrFeCo made from Q235 steel using laser cladding method. With increasing Co content, the coatings showed excellent corrosion resistance in H 2 SO 4 and HCl solutions. This was attributed to the increased Co content, more homogeneous organization of the coating and a denser passivation film on the alloy surface. Cyclic polarization curves showed that the Al 2 CrFeCo 2 CuNiTi alloy coating showed no corrosion at 0.5 mol/LH. 2 THEN 4 solution, while corrosion occurred in 0.5 mol/l HCl solution.
The corrosion resistance of alloy coatings in 1 mol/L NaOH and 3.5% NaCl solutions was also reported by Qiu et al. examined. The alloys also showed excellent corrosion resistance. The corrosion resistance of the alloys changed without a significant pattern as the Co content increased. Among them Al 2 CrFeCo 1.5 The CuNiTi alloy coating does not show corrosion in NaCl solution, but shows slight corrosion in NaOH solution.
1.1.6 Cr
Cr is an important element in stainless steel, has high potential and is easily passivated. Increasing its content improves the corrosion resistance of stainless steel. However, for some high entropy alloys the situation is different.
Li et al. produced AlCoCr The corrosion resistance of the coatings first increased and then decreased with increasing x in solutions of 0.1 mol/L HCl and 3.5% NaCl. The best corrosion resistance of the alloy was achieved at x=1.5; at x=1.0 and x=1.5 the coating consisted of a single-phase solid solution of FCC; and at x = 0.5, x = 0.75 and x = 2.0 the coating contained a small amount of the fine-grained phase FeAl 3 distributed among the dendrites in addition to the solid solution phase.
1.1.7 Various elements
The present study shows that the addition of Nb, W and Ta improves the corrosion resistance of high entropy alloys, while the elements Sn and B play different roles. The investigation of the corrosion resistance of FeCoNiCuSn X (x=0,0.02,0.03,0.04,0.05,0.07,0.09) alloys shows that in NaCl solution the alloy with 0.04 has the best corrosion resistance; in NaOH solution, the alloy has the best corrosion resistance with x = 0.03, and increasing the Sn content further increases and weakens the corrosion resistance of the alloy. Lee et al. investigated the effect of element B content on the corrosion resistance of Al 0.5 CoCrCuFeNiB alloy in H. 2 THEN 4 . They found that the corrosion resistance of the alloy decreases with increasing B content, which is related to the Cr, Fe and Co borides formed in the alloy.
1.1.8 Interaction of elements
The above studies refer to the effects of changes in the content of an element in the alloy. However, the role of a specific element in the alloy is generally influenced by other elements, and the resulting changes in the organizational structure of the alloy that affect the corrosion resistance of the alloy are more complex. Currently, more research needs to be done in this area.
Qiu et al. investigated the corrosion resistance of Al the element Ti. However, when Ti and Al are added to the alloy, the corrosion current increases, the corrosion potential decreases, and the corrosion resistance is significantly weakened. This is related to the precipitation of the Fe-Cr phase in the alloy after the addition of Ti.
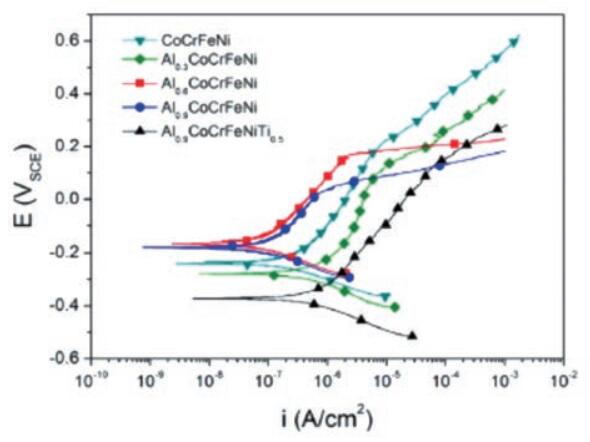
Fig.3 Polarization curves of Al X CoCrFeNiTi j Alloy in 0.6M NaCl solution
Wang et al. investigated the effect of the components of a high entropy single-phase CoCrFeNi alloy on its corrosion resistance in NaCl solution. For the same Co and Cr content, increasing the Fe content and decreasing the Ni content can reduce the dimensional passivation current density of this alloy system. For the same Fe and Cr content, increasing the Co content and decreasing the Ni content can also reduce the dimensional passivation current density of this alloy system, thus improving its corrosion resistance. With the same Cr content, reducing the Co content and increasing the Fe and Ni content can improve the self-corrosion potential of the alloy and reduce the corrosion tendency of the alloy.
1.2 The influence of the organization and structure of the alloy on its corrosion resistance
Heat treatment can change the microstructure and structure of metallic materials and adjust the distribution of alloying elements, which affects the corrosion behavior of alloys. Most of the related research works deal with highly entropic alloys of the AlCoCrFeNi system.
Al 0.5 casting alloy The matrix organization of CoCrFeNi alloy is a face-centered cubic structure. After aging treatment from 350°C to 950°C, the matrix organization transforms into face-centered cubic and body-centered cubic phases.
The polarization curves of the alloy in 3.5% NaCl solution after aging show that its corrosion resistance is worse than that of 304 stainless steel and cast alloys. This is related to the formation of AlNi and Al (Ni, Co, Cr, Fe) phases during the aging process, as shown in Figure 4. 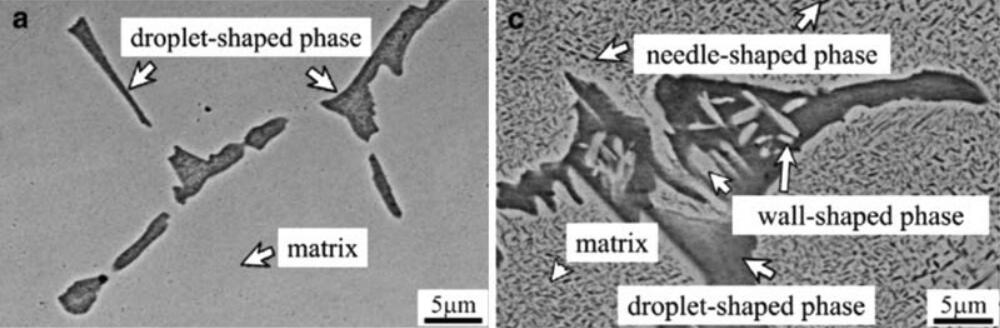
Fig. 4 Microstructure of Al 0.5 CoCrFeNi Alloy
(a) launch condition; (c) aging condition at 500°C
Liaw and Yang et al. Al homogenization heat treatment uniformity of elements in alloys as a result of heat treatment.
Jiang et al. compared the corrosion resistance of AlCoCrFeNi alloy samples in the as-cast condition with those in the annealed condition. The microstructure of AlCoCrFeNi alloy cast and annealed at 600°C and 800°C consisted of a single-phase solid solution of BCC in dendritic form; the dendrites disappeared from the alloy organization in the annealed state at 1000 °C, and the microstructure was transformed into two phases of BCC + FCC.
In a 3.5% NaCl solution, the corrosion resistance of the alloy is better in the annealed state at 1000 °C. In 0.5 mol/L NaOH solution, the corrosion resistance of the alloys in the four states did not differ much. In 0.5 mol/l H 2 THEN 4 Solution, passivation occurred in all four states of the alloy. In the 1000°C annealed state, the dimensional passivation current density of the alloy is the lowest, the rupture potential is the highest, and the corrosion resistance is the best, because in the 1000°C annealed state of the alloy, The Cr and Al distribution is the most uniform and in the weak areas.
There is no passivation film in the areas.
Zhang et al. investigated the change in corrosion resistance of AlCoCrFeNiTi 0.5 High Entropy Alloy after tempering at 600°C, 700°C, 800°C, 900°C and 1000°C. The cast AlCoCrFeNiTi 0.5 High entropy alloys consist of a simple body-centered cubic phase with ω phase.
As the tempering temperature increases, the grain grows and the eutectic organization increases. In 3.5% NaCl solution, the alloy undergoes porous corrosion, which is mainly concentrated at the interface of the dendritic crystals and in the α phase of the eutectic organization. The corrosion resistance of alloys after tempering is better than that of cast alloys, which is related to the reduction of disorganization defects after tempering.
The corrosion resistance of alloys after tempering at 700°C is stronger than that of the molten state and other states.
The corrosion resistance of AlCrFeCoNiCu alloy cast in 3.5% NaCl solution is better than that of 304 stainless steel. The corrosion resistance of the alloy decreases after annealing at 600 °C. However, corrosion resistance improves after annealing at 1000°C, when the alloy has the highest corrosion potential and the lowest corrosion current.
This is due to the rearrangement of the lattice atoms during annealing of the alloy at 1000 °C. Guo and Zhang et al. also investigated highly entropic alloys in this system, whose organization contains face-centered cubic phases and body-centered cubic phases. The corrosion resistance of alloys in 3.5% NaCl solution after annealing at 1000 °C is better than that of cast alloys. After annealing, the form of corrosion changed from intergranular corrosion and pitting corrosion to simple pitting corrosion, which was related to the reduction of the copper element segregation areas between the dendrites in the annealed organization.
Solid solution treatment of CoCrFeNiTi alloy by Fujieda et al. led to a more homogeneous composition. The corrosion potential in 3.5% NaCl solution has been increased and the corrosion resistance has been improved.
Wang et al. Mixed Crystal Treated Ni 2 CrFeMox Alloy: After holding at 1200°C for 1 hour, the σ phase in the alloy dissolved, the element distribution became more uniform, which limited the occurrence of galvanic coupling corrosion and significantly improved corrosion resistance.
Liu et al. prepared Al 2 NbTi 3 v 2 Zr alloys by low and high energy ball milling and subsequent vacuum hot pressing and investigated the corrosion behavior of the alloys in a 10% HNO 3 Solution.
The alloys prepared by high-energy milling showed better corrosion resistance than those prepared by low-energy milling. The corrosion resistance of the low-energy ball-milled samples depended on the content of the second phase, while that of the high-energy ball-milled samples depended on the grain size of the second phase and the matrix.
Cui et al. compared the kinetic potential polarization behavior of two FeCoNiCrAl alloys in two different solidification states in a 3.5% NaCl solution. The results showed that the corrosion resistance of the directionally solidified alloy was better than that of the non-directionally solidified alloy. This occurs because the former is more easily passivated and in directional solidification there is no transverse grain boundary between the initial growth and quenching interfaces.
2. Corrosion of high entropy alloys in high temperature media
Li et al. investigated the corrosion behavior of CoCrFeNiTi 0.5 High Entropy Alloy in molten Na 2 THEN 4 -25% NaCl medium. The corrosion kinetic curves of CoCrFeNiTi 0.5 alloys at 650°C and 750°C are similar and show an “exponential” growth pattern, as shown in Figure 5.
High-temperature corrosion of the alloy in Na 2 THEN 4- 25% NaCl medium is attributed to the combined effects of oxidation, sulfidation, and chlorination. The corrosion of the alloy at 650 °C and 750 °C in a solution with 0.75% SO 2 Atmosphere was also examined and the kinetic curves followed a parabolic pattern. Corrosion products consisting of Ti, Cr 2 Fe oxides, complex oxides structured in spinel AB 2 Ó 4 and sulfides (FeNi) were formed on the surface of the alloy.
High temperatures significantly increase the thickness of the oxide film and the density of the pores in the area affected by corrosion, resulting in a weaker bond of the oxide layer to the substrate or even in flaking and an increase in the depth of corrosion. Corrosion of CoCrFeNiTi 0.5
Corrosion of high-entropy alloys in sulfur-containing atmospheres is due to oxidation of alloying elements in the initial stage of corrosion, as well as subsequent sulfidation of metal oxides, formation of ternary eutectic composite salts, and dissolution reactions. of the alloy element Fe in the molten salt. 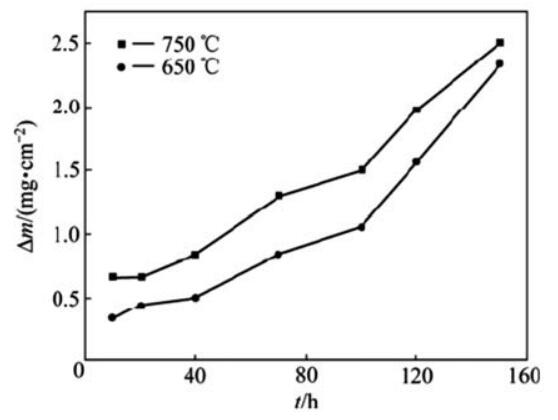
Fig. 5 Corrosion kinetic curves of CoCrFeNiTi 0.5 High entropy alloy at 650°C and 750°C
The oxidation properties of the FeCoNiCrMn alloy in a CO 2 /CO mixture at high temperatures were reported by Kai et al. examined.
The oxidation of the alloy follows a parabolic law at both temperatures, 700 °C and 950 °C. The structure of the surface oxides of the alloy is different at different temperatures.
The CoCrCuFeNiAl 0.5 b X alloy has FCC structure. At 500 °C, the alloy does not show significant corrosion in syngas with 0% and 0.01% H 2 S, while in syngas with 0.1% and 1% H 2 S, the alloy is subject to significant corrosion.
The polyphase Cu – Rich zone in low boron alloys is susceptible to corrosion, and Cu 1.96 S is the main sulfide for corrosion in low boron alloys. In alloys with high boron content, FeCo 4 No 4 S 8º is the main sulfide.
3. Corrosion of high entropy alloys in special media
The element content of high entropy alloys is generally high, and some elements are also very expensive compared to conventional alloys. The cost of high entropy alloys represents a major disadvantage, therefore studying the corrosion of high entropy alloys in extreme operating environments is of great practical importance.
Han et al. investigated the corrosion behavior of various high-entropy alloys in high-temperature, high-pressure nuclear power plants. 1.5 CrFeNi 1.5 Ti 0.5 Mo 0.1 The alloy has FCC structure, AlCoCrFeNiSi 0.1 The alloy and TaNbHfZrTi alloy have CCC structure. TaNbHfZrTi and Co 1.5 CrFeNi 1.5 Ti 0.5 are more expensive than conventional alloys. 0.1 have better stress corrosion resistance than AlCoCrFeNiSi 0.1 and conventional 690TT alloys.
The main components or cladding materials made of such high-entropy alloys have greater safety in the high-temperature and high-pressure aquatic environment of nuclear energy.
Yang et al. produced highly entropic AlCrMoNbZr/(AlCrMoNbZr)N alloy coatings with a face-centered cubic structure on Si substrates using a magnetron sputtering process.
Under high-dose He irradiation, coatings with a single layer thickness of 50 nm showed better interfacial stability, mechanical properties and corrosion resistance than AlCrMoNbZr/(AlCrMoNbZr)N multilayers with a thickness of 5 nm per layer. This study demonstrates the potential application of high-entropy alloy cladding as crash-resistant fuel cladding for light water reactors.
Csaki et al. investigated the corrosion of AlCrFeNiMn alloy in geothermal steam. The alloy has a body-centered cubic structure and the corrosion rate is about 3.25 mm/a in geothermal steam at 200 °C and pressure of 1.65 MPa. The main components of surface corrosion products are MnS and Ni. 2 S 3 . It can be seen that the high entropy alloy is not suitable for geothermal steam rich in H 2 S and CO 2 .
4. High entropy air bubble corrosion alloy
When liquid and metallic components move relative to each other at high speeds, a vortex is created locally on the metal surface.
In this context, bubbles form quickly and burst on the metal surface, presenting damage characteristics similar to pitting corrosion (known as blister corrosion).
Grewal et al. investigated the cavitation corrosion resistance of Al 0.1 CrCoFeNi alloy in NaCl solution.
The alloy structure is a face-centered cubic structure, and its cavitation resistance is better than that of 316 stainless steel. This is related to Al. 0.1 CrCoFeNi alloy with better work hardening ability and more stable surface passivation film .
Zhang et al. produced FeCoCrAlNiTi X Alloy coatings on 304 stainless steel by laser melting.
In distilled water, the cavitation resistance of the coatings increased with increasing Ti element content, which was mainly due to the hardness and uniform distribution of the two intermetallic compounds Ti. 2 Ni and NiAl, which showed better resistance to deformation and higher strength to fracture.
However, in 3.5% NaCl solution, the cavitation resistance of the alloys weakened with increasing Ti element content, which may be due to the weakened passivation stability of Ti. 2 Ni and NiAl in the ion environment chloride.
5. Application of phase diagram calculations and kinetic simulations to study the corrosion resistance of high entropy alloys
Zhang et al. used the CALPHAD thermodynamic method (phase diagram calculation) to predict the matrix phase structure and corrosion behavior of the CoCrFeNi-based high entropy alloy system.
CALPHAD simulation can also generate the Pourbaix diagram of the alloy, determine the corrosion zone, passivation zone and corrosion resistant zone of the alloy, and the corrosion behavior of the alloy can be evaluated in the medium with different pH values, as shown in Fig. 6.
As shown in Figure 6. Lu et al. also used the CALPHAD method to design Ni 38 Cr 21 Fe 20 ru 13 Mo 6 b 2 Alloy whose excellent corrosion resistance has been experimentally proven. 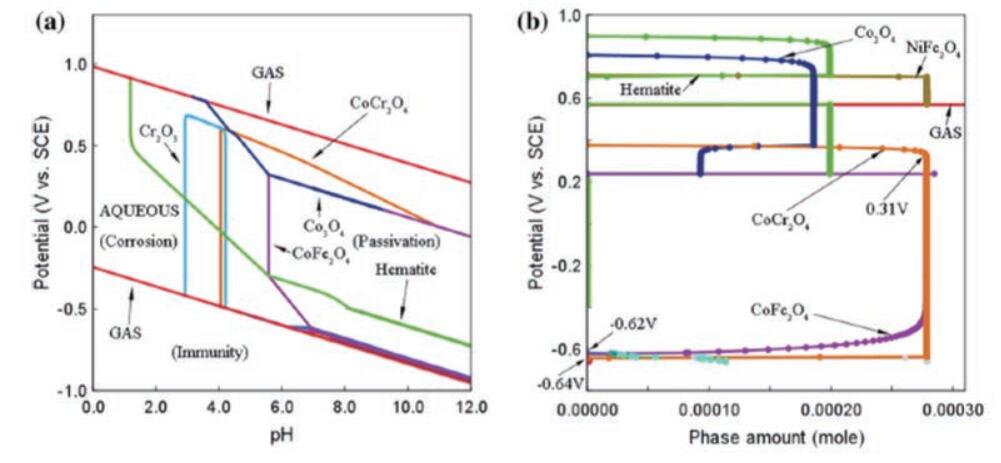
Fig. 6 Corrosion behavior of CoCrFeNi alloy
(a) Pourbaix diagram; (b) Phase characterization diagram at pH=7
Song and Liaw et al. experimentally investigated the effect of Cr and Ti addition on the microstructure and corrosion properties of AlCoCuFeNi alloys in combination with molecular dynamics simulation methods.
The addition of Cr to the alloy promotes the formation of a solid CCC solution in the alloy. The addition of Ti promotes the formation of the FCC phase. The addition of Ti reduces the corrosion resistance of the alloy, while the addition of Cr improves the corrosion resistance of the alloy without Ti.
6. Comparison of corrosion resistance between high entropy alloys and conventional alloys
Currently, most corrosion tests of high entropy alloys are carried out in two media: an aqueous NaCl solution or H 2 SO 4 aqueous solution.
In NaCl solution, most forms of alloy corrosion are pitting corrosion. As shown in Figure 7, the pitting corrosion potential of high entropy alloys is much greater than that of Al alloys, Cu alloys and some Ti alloys, and comparable to that of stainless steel and Ni alloys. The corrosion current density of high entropy alloys is much lower than that of Cu and most Ti alloys.
In H 2 THEN 4 Solution: The corrosion current density of high entropy alloys is lower than that of most steels, and their corrosion potential is higher than that of steels, titanium alloys, nickel alloys, and most steel alloys. copper, as shown in Fig. 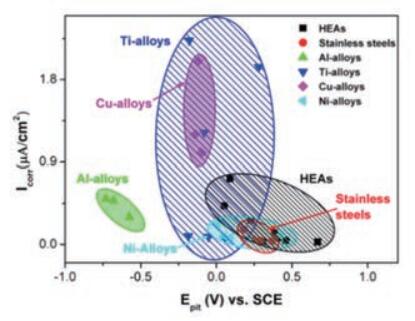
Fig. 7 Comparison of corrosion current density and corrosion potential of high entropy alloys and conventional alloys in a 3.5% NaCl solution 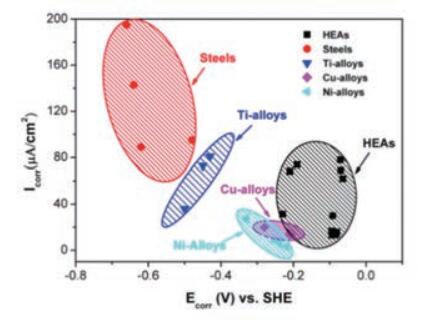
Fig. 8 Comparison of corrosion current density and corrosion potential of a high entropy alloy and a conventional alloy at 0.5 MH 2 THEN 4 Solution
It can be seen that high entropy alloys are more resistant to chloride ions and acids than many conventional metallic materials. Furthermore, high entropy alloys can combine excellent toughness and corrosion resistance, optimizing the organizational structure.
7. Conclusion
This article briefly reviews some advances in the study of corrosion resistance of high entropy alloys and draws the following conclusions.
- (1) The corrosion resistance of high-entropy alloys can be continuously improved by selecting effective alloying elements and adjusting the organizational structure of the alloys through manufacturing and heat treatment processes.
- (2) High entropy alloys have good application prospects in extreme operating environments.
- (3) The application of first-principles methods and molecular dynamics simulation is an effective way to accelerate the exploration of the properties of high-entropy alloys.

























































