Despite initially having a higher price, electric vehicles (EV) tend to offer significant savings over time. Leaving aside the environmental benefits, which are noteworthy, EVs typically cost half the maintenance and repair price of gas-powered vehicles – and their popularity is growing.
Unlike conventional vehicles, EVs do not have internal combustion (IC) engines. Instead of gasoline, they run exclusively on batteries. For some drivers, the concern was that this meant a lack of decent torque. However, this is no longer the case, and many electric vehicles boast power and torque similar to that of an average IC engine.
What's more, battery life is gaining longevity. Most are expected to last five to eight years. The battery is the heart of EVs.
Batteries
Battery technology for electric vehicles has developed from the first lead-acid batteries used in the late 19th century, until about 2010, to lithium-ion batteries. Lithium (Li-on) batteries, the same technology that powers our smartphones and tablets, are currently found in most electric vehicles.
Li-on batteries have been revolutionary since their creation in the early 1990s, offering high charge levels compared to other technologies. They are also lightweight, temperature tolerant, fast charging, and offer decent shelf life and shelf life.
In terms of EVs, six lithium-ion battery technologies are popular among manufacturers. Let's explore all these battery chemicals.
Why lithium-ion batteries?
Lithium-ion batteries offer a high energy density that allows them to store a large amount of energy in a small space, making them the most popular choice for most electric vehicles. These batteries also provide a high power-to-weight ratio, meaning they are lighter and typically more efficient than other types.
Perhaps most importantly, lithium-ion batteries are safer than alternatives, providing good performance at high temperatures. They offer high efficiency with low self-discharge.
A lithium-ion battery is made up of several lithium cells. Cells are arranged in series and parallel at the desired voltage and current capacity. For example, if a 12.8V 125AH battery includes 3.2V 25AH lithium-ion cells, 4S5P is the required configuration. This means that five cells are connected in parallel for a 125 AH output in each master pack, and four master packs are connected in series for 12.8V.
In an electric car, hundreds of lithium-ion cells are used to build the battery. In the Tesla Model S 444 Panasonic, the NCR18650B cells are connected in a 6S74P configuration.
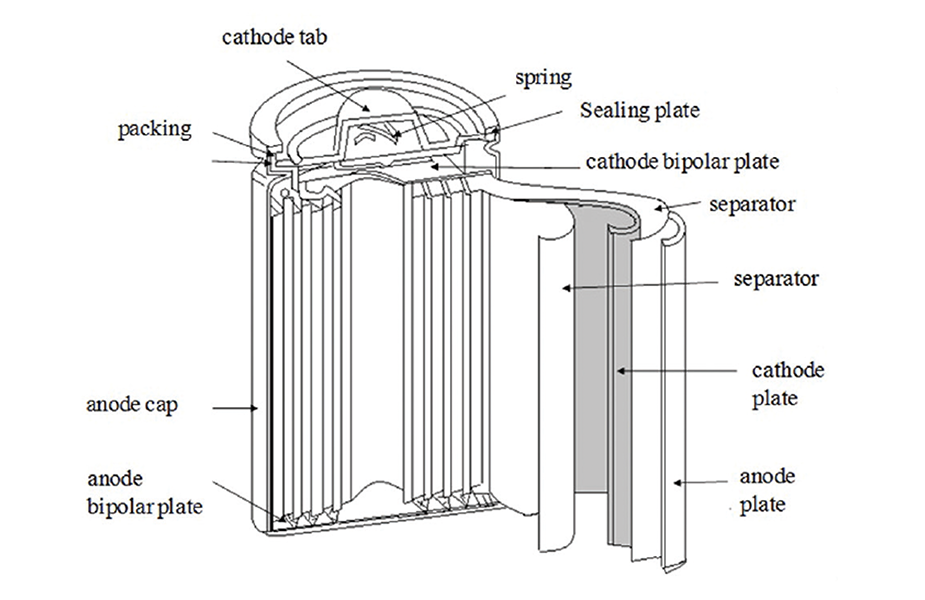
The lion The cell is the building block of a battery. These cells come in cylindrical, prismatic and pouch formats used to build EV batteries by Tesla and Panasonic. Most other manufacturers use pouch or flat cells. Prismatic cells are also used, but by few manufacturers. The main components of a lithium-ion cell are the cathode, anode, electrolyte and separator.
• The cathode is the workhorse of a lithium battery and the active material of a lithium metal oxide. Lithium is highly reactive, so it is mixed with oxygen and metal for stability. The metal used determines the voltage output of the battery. The current capacity of the battery is determined by the size of the cathode. A battery with a larger cathode has greater charge storage. Li-on batteries are named after the lithium metal oxide used as the cathode.
• The anode is the battery's second electrode, responsible for storing the lithium ions that charge the battery. The current capacity of the battery is determined by the surface area of the anode, which must have high porosity and conductivity to function effectively. The anode voltage must match that of the cathode and the surface area of the anode is maximized to create a higher current.
Typically, graphite-coated copper foils are used as anodes in lithium batteries because the graphite matches the voltage. Typically, amorphous carbon, synthetic graphite and natural graphite are used as carbon-based anode materials. Manufacturers sometimes add silicon to carbon to increase the battery's energy density. Another material sometimes used is lithium titanium oxide, known for its durability and thermal stability.
• The electrolyte is an electrically neutral solution in which both electrodes are submerged. Although the electrolyte is non-reactive, it acts as a catalyst that makes the battery conductive during charging or discharging. Facilitates the movement of ions in both processes.
When a lithium-ion battery is charged, ions flow from the cathode to the anode through an electrolyte. When the battery discharges, this flow of ions passes from the anode to the cathode again through an electrolyte. Anions and cations move in opposite directions during charging and discharging, but only when there is a difference between the cathode and anode. Lithium salt is used as an electrolyte in lithium-ion batteries, such as LiPF6 in an organic solvent.
• Separator is used to isolate the cathode and anode. When there is an applied difference between the two, ions flow through the electrolyte between them. This potential difference also forces electrons to move from the anode to the cathode. The separator is responsible for blocking electrons, only allowing ions to move through the electrolyte. The separator is covered with electrolyte to allow the movement of ions.
The separator also acts as a fuse for the battery. But it can get clogged due to melting due to overheating. If this happens, the current flow between the cathode and anode will stop before the battery catches fire. Lithium-ion batteries use polyolefin as a separator. In large batteries, a three-layer separator is used.
The conductive plates collect the current between the cathode and anode of the battery. A copper plate is used as a current collector at the anode. An aluminum plate is used as a current collector at the cathode.

Types of Lithium-Ion Batteries
Lithium-ion batteries are classified by their cathode and use a graphite anode. The only exception is lithium titanate (LTO). The use or consideration of different cathode materials represents an effort to maximize energy density. For example, researchers are currently evaluating the use of lithium metal as an anode material and replacing the use of lithium salt as an electrolyte with a solid-state electrolyte.
But for now, lithium-ion batteries differ only in the cathode material and use the same anode and electrolyte materials. Here are the top six lithium-ion battery chemistries used in the EV industry.
1. Lithium-Cobalt Oxide (LCO)
2. Lithium Manganese Oxide (OML)
3. Lithium Iron Phosphate (LFP)
4. Lithium-nickel-cobalt-manganese oxide (NCM)
5. Nickel Lithium Cobalt Aluminum Oxide (NCA)
6. Lithium Titanate (LTO)
Lithium-Cobalt Oxide or LCO-based batteries were the first Li-on batteries in commercial production. Invented in 1991, CO-based Li-on batteries quickly gained popularity in consumer electronics due to their high energy density (150~200 Wh/Kg). However, the disadvantage of these batteries is that they have low thermal stability and are prone to overheating. This means they pose a fire risk at high temperatures or if they are overloaded.
Lithium Manganese Oxide or LMO-based batteries were first introduced in the mid-1990s as an alternative to LCOs, offering lower internal resistance. LMO-based lithium batteries were important because they offered greater thermal stability and a lower risk of overheating. What's more: they delivered higher currents, ranging from 20 to 30 Amps. Higher current levels also mean faster charging and discharging.
These batteries quickly became the best choice for power tools, power banks, and eventually EVs. The C rate for LMOs is 2/5 compared to 2/3 for LCO batteries. This means that if an LMO-based battery takes two hours to fully charge, it will take five hours to discharge. But there is one disadvantage they have in common with LCO-based batteries: the low cycle life (500 to 700). Therefore, neither option is ideal for long-term applications.
Lithium Iron Phosphate or LFP-based batteries have been launched as a means of solving the low cycle life of LCO and LMO-based options. Chemists were determined to find a better solution, and they found one in 1996. LFPs offer a lower energy density (90~160 Wh/Kg), but a cycle life of 4000. C rates can be 2/5 , the same as batteries based on LMO – or higher and 2/30. LFP-based batteries have high thermal stability and can easily withstand mechanical disturbances. The only disadvantage is that its nominal voltage is limited to 3.2V. But due to their high cycle life, low cost, reliability and thermal stability, LFPs were quickly adopted as the Li-on batteries of choice by the automotive industry. They are still used in e-rickshaws, electric bicycles and various power tools.
Lithium-nickel-cobalt-manganese oxide or NCM-based batteries comprise 60% nickel, 20% cobalt and 20% manganese as active materials in the cathode. This battery chemistry was invented in 2001, offering one of the highest energy densities available for lithium-ion (150~220 Wh/Kg). These batteries offer good thermal stability, high nominal voltage (3.7V) and a decent cycle life (2000 at 1/3 C rating).
Furthermore, the energy density and power supply of these batteries can be adjusted by changing the proportion of nickel, cobalt and manganese in the permitted active material. NCM-based lithium-ion has quickly become the preferred choice for electric cars and is widely used in medical equipment and power tools.
Lithium Titanate or LTO based batteries made their debut in 2012, presented by YABO Power Technology. Based on nanotechnology, LTOs have a lithium titanate anode instead of graphite. Lithium titanate offers a larger surface area, allowing for a high charge and discharge rate. The C rating of these batteries is 10/30, so once fully charged, the battery can last up to 30 hours. LTO-based batteries offer the longest cycle life (30,000) of all Li-on batteries. Its disadvantage includes low energy density (50~80 Wh/Kg) and low nominal voltage (2.4V). Despite the facts, several automotive manufacturers have considered LTOs due to their high cycle life and slow discharge rate. These batteries are already used to store renewable energy.
Lithium-Ion Chemistry in Automobiles
Li-on batteries based on NCA, NCM, LCO and LMO have a nominal voltage of 3.6/3.7 V, while LTOs offer the lowest nominal voltage of 2.4 V. LTOs also have the energy density lowest of 50~80 Wh/Kg. But LTOs batteries provide the highest cycle life of 30,000 with a discharge rate of 30 hours.
LTO-based batteries are widely used to store renewable energy. However, due to the charging time of up to 10 hours, high cost and low energy density and nominal voltage, its application in EVs is questionable. LCO-based battery chemistry is quite outdated. Even OVMs have only 1% usage in the automotive industry.
NCAs offer the highest energy density, but due to the low cycle life of 1,000, they are not widely used in electric vehicles. LFPs account for almost a quarter of usage in the EV industry due to their high life cycle of 4000 and a C-rate of 2/30. This means that an EV with an LFP-based Li-on battery that is only charged for two hours can run for about 30 hours. With a cycle life of 4,000, even if the EV is charged daily, the battery can be guaranteed for at least five to eight years.
Currently, NCMs are the most used Li-on batteries in electric vehicles, with 60% of the market. These batteries have half the cycle life of LFPs, but almost double the energy density at 220 Wh/Kg (compared to just 120 Wh/Kg). Higher energy density supports a higher power-to-weight ratio in EVs. Furthermore, the C rate of NCMs is 1/3. NCM's battery chemistry also features high thermal and mechanical stability, so they make sense as the best choice for EVs in today's market.