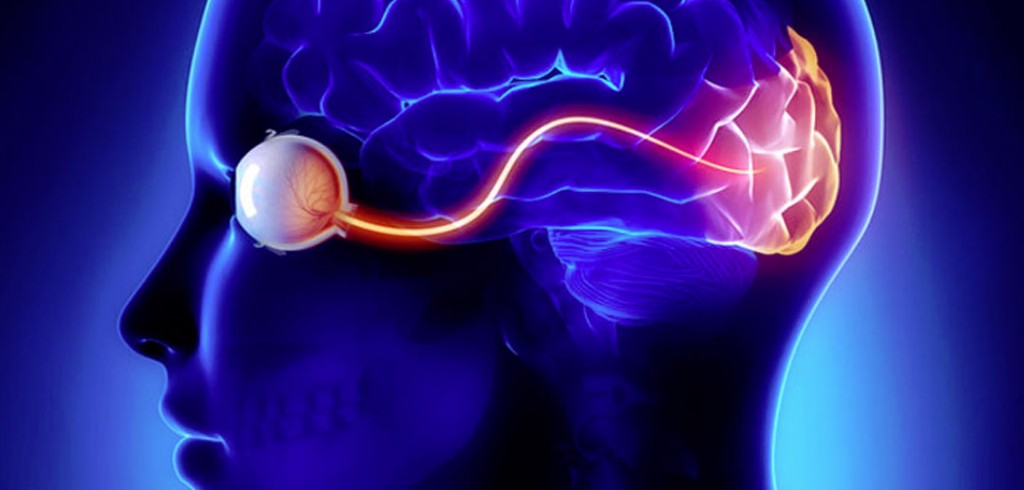
Recent advances in neuroscience and neurotechnology promise to restore functional vision to the blind by sending signals directly to the brain's visual cortex. Similar to the cochlear implant that worked wonders for the deaf, research is underway to restore visual perception to the blind through an implant that works by stimulating the visual cortex while trying to recreate the activity previously triggered by the optic nerve.
 The implant is designed to create the sensation of seeing even without actual eye input. Transmitting video images from a camera directly to the brain, the device targets the brain instead of the eyes, giving blind patients the ability to partially understand their surroundings.
The implant is designed to create the sensation of seeing even without actual eye input. Transmitting video images from a camera directly to the brain, the device targets the brain instead of the eyes, giving blind patients the ability to partially understand their surroundings.
In fact, visual prosthetics have undergone rapid advancement since their first prototypes. They have the ability to artificially encode sensations in the brain to produce points of light, or phosphenes, in the visual field.
How vision- helping implants work
Simply put, visual prosthetic technology works in the following ways to restore vision, however fractional, to the blind:
- A camera embedded in a pair of thick, black-rimmed glasses records the patient's field of vision.
- This camera, connected to a computer, sends live video that the computer translates into electronic signals.
- These signals are then sent through a surgically inserted port in the back of the skull. This port connects to a 100-electrode implant in the brain's visual cortex.
- The implant stimulates neurons in the patient's visual cortex.
- Subsequently, the patient experiences a low-resolution perception of the environment in the form of yellowish-white dots and shapes called phosphenes that can be interpreted as objects.
Evolution of vision restoration technology
The focus of previous research into bioengineering technologies was on creating an artificial eye or retina. Not long ago, the Artificial Retina Project brought together several research institutions to develop artificial retinas to help the blind see. The work resulted in the Argus systems that helped those suffering from retinal-destroying diseases. These systems involved the use of a camera mounted on glasses; a computer to convert sensory data and an implant with a set of electrodes embedded in the retina (instead of the brain). Over the years, the Argus I and Argus II systems have undergone human testing and received approval in Europe in 2011 and the US in 2013 to sell their bionic eyes to eligible individuals.
The implant comprised a single sheet of retinal pigment epithelial (RPE) cells derived from human embryonic stem cells. However, attempts to create a bionic eye have focused on implanting it into the eye itself. They depended on the need for an eye with a functioning optic nerve. But for many people whose blindness originates beyond the retina, artificial eye technology cannot resolve their blindness. These people suffer damage to the nervous system that connects the retina to the back of the brain or to the neural pathways between the eyes and the brain.
Progress in brain implants
Brain-Machine Interface (BMI) is advancing rapidly on several fronts, such as helping paralyzed people control robotic arms and typing messages with just their thoughts. Companies working in the BMI domain have moved from artificial retinas to the brain itself. They tested a system that bypasses the retina and sends visual information directly to the brain.
In this system, as previously mentioned, transmission from a video camera mounted on sunglasses is converted into electrical pulses sent to an implant that stimulates the brain. Subsequently, the user sees a pattern of low-resolution white phosphenes or dots on a black background.
The Utah array, a widely used brain implant, is a square array a few millimeters wide that contains 100 electrode tips that are inserted into the brain. Each spike stimulates neurons in the brain. The array is slightly smaller than the raised tip on the positive end of a AAA battery. Tiny electrode spikes, each about a millimeter tall, look like a miniature bed of nails. Each spike is capable of supplying current to one or four neurons.
The implant that transmits video images directly to the brain is truly a breakthrough. The video camera attached to the glasses sends images to electrodes implanted in the brain's visual cortex, allowing participants to experience some level of vision where previously not possible.
The technology is notable because it bypasses both the eye and the optic nerve, which would normally transmit sensory information to the brain.
A case study on brain implantation
Neuroengineer Eduardo Fernandez, director of neuroengineering at the University of Miguel Hernandez in Spain, recently made news for empowering a blind woman with rudimentary vision by sending visual information directly to her brain.
The patient in question, Bernadeta Gomez, aged 57, had been completely blind since the age of 42, when she began to suffer from toxic optic neuropathy, a condition that completely severed the connection between the eyes and the brain.
Fernandez implanted a tiny chip in Gomez's brain, containing 100-millimeter-long electrodes that stimulated specific neurons that allowed him to see a close-up picture of the world for the first time in 16 years. She saw the world through phosphenes – an experience of seeing light without being triggered by retinal stimulation, which gave her a faint perception of shapes and space around her with an approximate resolution of 10×10 pixels.
To compensate for the poor resolution, Fernandez incorporated facial recognition software that helped her associate the set of corresponding phosphenes she received through the device with specific people. The device included a belt with a button to amplify dark objects in the sun or light objects in the dark.
The system allowed the woman to have “rudimentary” vision, despite the destruction of the nerve bundles that connected the eyes to the brain. She was able to see a low-resolution version of what the world is like. The glasses made it possible to identify letters, lights and people. It appeared looking like whitish and yellowish glowing dots and shapes, but it was at least an appearance of the world rather than pure darkness.
Challenges in restoring vision
The implant tends to deteriorate due to natural processes in the body. The body's immune system begins to break down the electrodes that damage or scar the surrounding brain tissue. Scarring eventually weakens the signal and the electrodes can only interact with a handful of neurons, eventually rendering them useless. The prosthesis can be left in the brain for a short period of time, as no one knows how long the electrodes can last without degrading the implant or the user's brain.
Another disadvantage is that when the implant is inserted, the electrodes pierce the surface of the brain; when it is removed, 100 small drops of blood form in the holes.
The road ahead
Fernandez's research points the way for other ambitious projects that aim to bridge the gap between computers and humans using brain-computer interfaces (BCI). Significantly, BCI aims to change the quality of life for people with disabilities. Britain's Royal Society has called for more attention to be paid to the development and regulation of BCI, saying the technology has the potential to usher in a "new, collaborative form of intelligence."
Fernandez's process also relies on more than just hardware. It deploys machine learning to write software that translates visual information into neural code that can be further refined. In the future, as scientists work on brain implants as a whole, the components will undoubtedly improve in parallel.
Fernández believes his implant can be modified to last longer, and although the current maximum resolution is 10 by 10 pixels, he aims to achieve a resolution of at least 60 by 60 pixels by implanting up to 6 electrodes on each side of the brain.
Researchers at Harvard Medical School have been working on a new type of implant that is not affected by scar tissue. Eliminating the need to penetrate the organ, these new electrodes are placed below the skull to rest on the surface of the brain. Additionally, powerful magnetic fields are used instead of electrodes to induce brain activity.
SpaceX and Tesla CEO Elon Musk's ambitious Neuralink project aims to develop implants that insert directly into neurons and provide two-way communication between the brain and a smartphone app. He wants to bring computing to people with severe loss of brain function. To achieve better accuracy, he wants the surgical implants to not be large and to be completed by a robot. Soft thread-shaped electrodes are being developed by Neuralink to be able to be skillfully tied to brain tissue by a robot. The organization aims to include 3,000 electrodes in its device to connect many more neurons than is currently possible.
In fact, more and more new technologies are being developed in the field of visual prosthetics. Typically, vision glasses are connected to power, the computer, and the implant via cables. The ideal device needs to be wireless so that it can last a long time – limiting the number of surgeries required – and offer greater precision and resolution. It could do away with electrodes altogether, using light or chemicals to control gene-edited neurons.