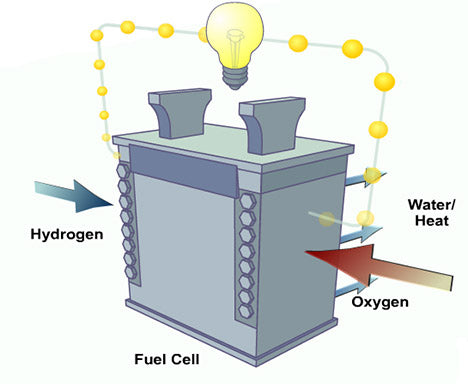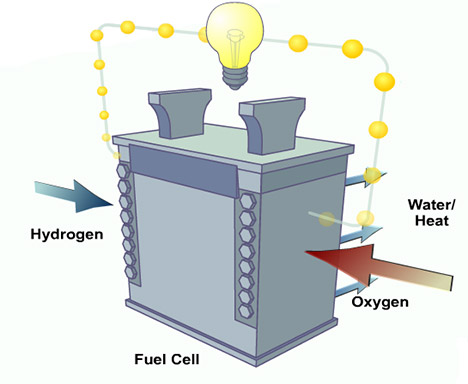
Fig. 1: Simple Diagram Showing a Fuel Cell
The first law of thermodynamics states: “Energy can neither be created nor destroyed, but it can be converted from one form to another.” The first part of this law tells us that there is a fixed amount of energy in the universe, while the second part gives an idea of how we can use the available energy to change it into a form that is useful to us. The ideal case would be for the entire portion of the source's energy to be converted into the form of our choice. For example, a perfect gasoline car would produce energy exactly equal to the energy released by breaking the bonds of hydrocarbon fuel through combustion, which would mean a 100% efficient machine. Unfortunately, we do not live in such a perfect universe and such a machine (called a perpetual motion machine of the first type – PMM 1) does not exist.
There are always losses when it comes to energy conversion, whether due to friction, heat or vibration. Which means that the energy from the source is not exactly converted into just one form of energy. Therefore, engineers do not try to eliminate losses when designing machines, but they try to minimize losses as much as possible, using the second law of thermodynamics which deals with the concept of “Entropy”. Basically, this law explains the directionality of energy transfer by dividing energy into two categories, high-grade energy and low-grade energy. A high-grade energy can be easily converted to a large extent into a low-grade energy, but the reverse is more inefficient. Considering the example of a gasoline engine in a car, the chemical energy of gasoline is first converted into thermal energy (lower grade energy) by combustion and then into mechanical energy (higher grade energy) leading to low efficiencies (about 25% to 30%). ). If we could eliminate the conversion process to lower quality energy, we could create a more efficient energy device. This is exactly the purpose behind a “Fuel Cell” that directly converts chemical energy into electrical energy. In this article we will understand how a fuel cell works, its different types and their applications.
The Fuel Cell – Working
The fuel cell is basically an electrochemical energy conversion device that works according to the opposite principle to “Electrolysis”. In electrolysis, water is broken down by applying electricity into hydrogen and oxygen, while in a fuel cell hydrogen gives up its electrons to oxygen and forms water. The path taken by electrons is directed through a circuit that produces electricity. Unlike batteries, which are also electrochemical energy devices, fuel cells can continue producing electrical energy constantly as long as fuel is supplied.
The two components Hydrogen and oxygen are separated by a membrane that does not allow electrons to pass through. Hydrogen passes through this membrane that separates the positive hydrogen ion from the electron and passes through an electrolyte solution towards the cathode section. The electrons that cannot pass through the membrane pass through the conductor to the cathode section and form a negative oxygen ion. The positive hydrogen ion and negative oxygen ion combine to form water which is released as a byproduct. The basic hydrogen fuel is supplied through pressurized gas containers and oxygen from the air. The pressurized hydrogen container is a rather heavy installation and therefore there are certain fuel cells that are designed to run on hydrogen-containing gases such as clean natural gas or renewable biogas.
Types of fuel cells
Types of fuel cells
The electrolyte used is different for different applications and operate at different temperatures. Based on this, fuel cells are classified into different types.
-
Polymer exchange membrane fuel cells (PEMFCs): These are probably the most commonly used fuel cells, as they utilize a polymeric membrane that contains the electrolyte solution between the electrodes and also operates at a relatively low temperature of about 60 to 80 degrees on the Celsius scale. A commonly used membrane material is Nafion, which is a hydrocarbon-based polymer. Hydrogen gas is forced through a platinum-coated catalyst that splits electrons and positive ions. Sandwiched fuel cells are usually stacked to provide the required output. They can also be used for smaller, more portable applications.
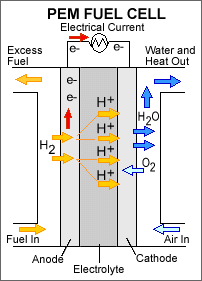
Fig. 2: Polymer Exchange Membrane Fuel Cell
-
Solid Oxide Fuel Cells (SOFCs): They are best suited for stationary power generation plants as they operate at higher temperatures of around 700 to 1,000 degrees Celsius. It uses a solid electrolyte made from a ceramic material called Yttria Stabilized Zirconia. They are unique from the other types in that they have negative oxygen ions traveling through the electrolyte toward the hydrogen ions. The high temperatures associated with this can be used in a cogeneration plant, where the waste heat from this cycle can be used to produce steam to power a turbine-driven generating plant.
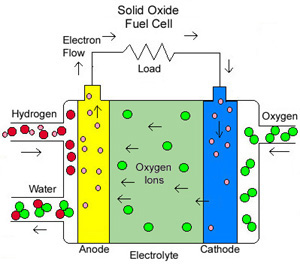
Fig. 3: Solid Oxide Fuel Cell
-
Alkaline Fuel Cells (AFCs): This type of fuel cell is one of the oldest designs made for the Apollo space programs to generate electricity as well as water for astronauts in space. It uses pure hydrogen and oxygen and is therefore very expensive. Alkaline solutions like Potassium Hydroxide (KOH) or Sodium Hydroxide (NaOH) are used as the electrolyte through which pure hydrogen and oxygen are bubbled. Operates in a temperature range of 70 to 140 degrees Celsius.
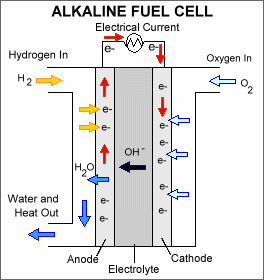
Fig. 4: Alkaline Fuel Cell
-
Molten carbon fuel cells (MCFCs): Like SOFCs, this type of fuel cell also operates at a high temperature of around 650 degrees Celsius. It uses lithium potassium carbonate salt as electrolyte in molten form. The advantage of this fuel cell is that it can extract hydrogen from general fossil fuels instead of using pure hydrogen gas. Keeping carbon emissions to a minimum.
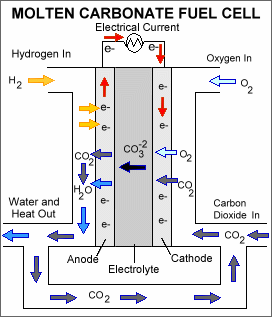
Fig. 5: Molten Carbon Fuel Cell
-
Phosphoric Acid Fuel Cells (PAFCs): These types of fuel cells use phosphoric acid as an electrolyte to pass hydrogen ions, but at the same time it is a non-conducting electrolyte. Operating temperatures are around 150 to 200 degrees. Although these temperatures are not ideal for a power plant, they are suitable for powering air conditioning plants. Although higher temperatures lead to longer warm-up time, making it unsuitable for automobiles.
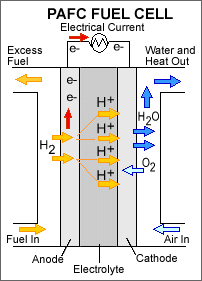
Fig. 6: Phosphoric Acid Fuel Cell
-
Phosphoric Acid Fuel Cells (PAFCs): These types of fuel cells use phosphoric acid as an electrolyte to pass hydrogen ions, but at the same time it is a non-conducting electrolyte. Operating temperatures are around 150 to 200 degrees. Although these temperatures are not ideal for a power plant, they are suitable for powering air conditioning plants. Although higher temperatures lead to longer warm-up time, making it unsuitable for automobiles.
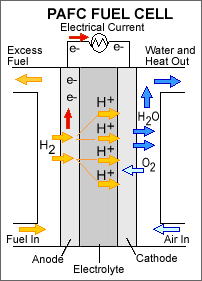
Fig. 7: Phosphoric Acid Fuel Cell
Direct Methanol Fuel Cells (DMFCs): This is a type of proton exchange fuel cell that uses methanol as a fuel source to supply hydrogen. Its operating temperature is similar to that of PEMFCs, but it has lower efficiency. It can be used in portable devices that require good power density rather than efficiency.
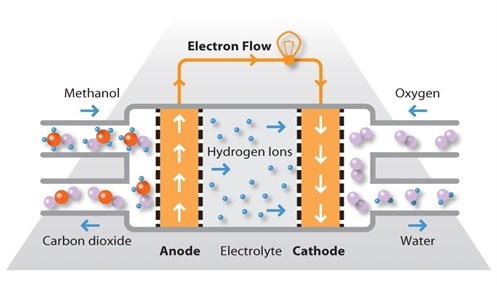
Fig. 8: Direct Methanol Fuel Cell
Enzymatic and microbial biofuel cells (EFCs and MFCs): Enzymatic and microbial biofuel cells fall into the special category of biofuel cells that use biological media as catalysts to release electrons. EFCs specifically use enzymes for this purpose, which are produced by living cells that are easy and cheap to mass produce, while MFCs use actual living organisms (microbes). These replace the use of expensive materials such as platinum and nickel. Enzymes and microbes are capable of breaking down simple organic molecules such as sugars, human waste, biofuels, etc. Biofuel cells have not yet found a way out of research laboratories, but they are very promising if they are commercialized taking into account their strong economic potential. They aim to find application in powering bio-implants, spacecraft life support systems, etc.
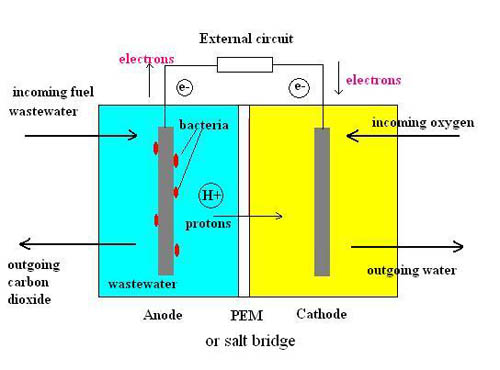
Fig. 9: Enzymatic and microbial biofuel cell
Application
Application Highlight – Fuel Cell Vehicles
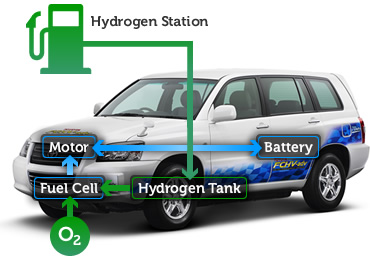
Fig. 10: Fuel cell implementation in a four-wheel vehicle

Fig. 11: Image showing the fuel cell on the hood of a car
The automotive sector has been the most impacted by fuel cell technology. Fuel Cell Vehicles are a common research interest among the big names in the automobile industry. The electricity obtained by the fuel cell is used to power an engine which is used to provide torque to provide rpm to the transmission system.
The electrical energy to mechanical energy of the engine carries an efficiency of 80%, which leads to an overall efficiency (80% of the fuel cell + 80% of the engine) of about 64% (although there are certain transmission losses) which when Compared to the 30% efficiency of gasoline engines, this represents a huge increase. These numbers are for a fuel cell that uses pure hydrogen.
As of 2015, there are two commercially available models, the Hyundai IX35 FCEV and the Toyota Mirai, although they were released in limited quantities. There are many other concept cars launched by automobile companies like Honda (Clarity FCX and FCV concept), Audi (A7 h-tron Quattro), Mercedes Benz (F-Cell and F800), Volkswagen (Golf Hymotion) and Nissan (TeRRA FCV SUV ). The most efficient models include the use of pure hydrogen which can add weight and therefore sometimes hydrogen containing compounds are used which add an additional device called a “Reformer” to extract hydrogen.
A question may still arise as to why battery-powered cars are not good enough to do the job? Although batteries are more efficient than fuel cells (around 90%), they cannot produce their own energy. The electricity used to recharge the batteries has to be obtained somewhere and this energy may not be produced in an ecological or efficient way.
Why fuel cells?
The main reason fuel cells are promising in power generation is that they are environmentally friendly. The main byproduct of fuel cells is water, which is another big advantage. As the transition to lower quality energy, such as thermal energy, is being left aside, they are highly efficient, up to 80% and up to 90%, that is, 80% of the energy released by the chemical reaction is converted into electricity.
Dependence on fossil fuels must end so that life can sustain itself comfortably on Earth and this is why scientists and engineers are working to find better ways to produce energy. Fuel cell technology may be the answer, but currently this technology is not strong enough to replace conventional energy production. They have questions related to cost, durability, storage, and other considerations. Countries are also working to produce hydrogen in an environmentally friendly way and have formed the “International Partnership for the Hydrogen Economy” between 17 countries. Hydrogen is the most abundant element present in the universe and powers the stars. When we use the gifts of nature, we must follow nature's rules.