Thanks to its excellent corrosion resistance, 304 stainless steel finds wide application in equipment and parts that require good comprehensive properties such as formability and corrosion resistance. It is widely used in various industries, including chemical equipment, pressure vessels, among others.
Related Reading: Stainless Steel Grades
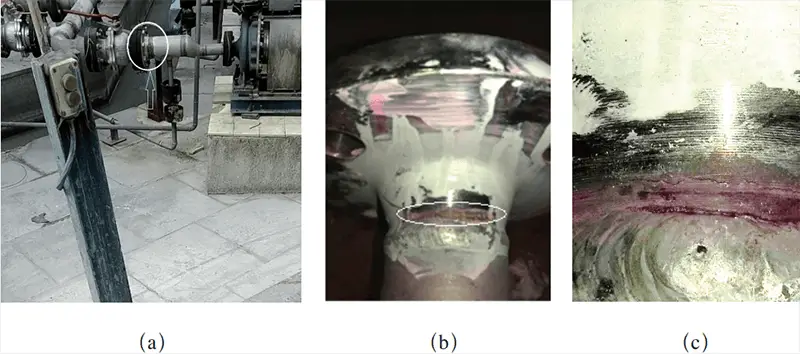
The sulfuric acid piping in a fertilizer plant connects the outlet (0.82 MPa) of the sulfuric acid pump to the reactor.
The pump has a flow rate of 14 m3/h, a head of 63 m, a sulfuric acid concentration of 93.5% and operates at normal temperature.
The piping was replaced in 2016, but after two years of use, liquid leaked from the weld of the discharge piping at the pump inlet and outlet, as well as from the high neck flange at the pressure gauge interface.
After cleaning and penetration testing the pipe wall, cracks were discovered (see Fig. 1).
Based on the original data, the steel pipe is made of 304 stainless steel, has a diameter of DN50 and a wall thickness of 3.5 mm.
After welding, the penetration test was carried out and the result was satisfactory.
Upon cutting and sampling the steel pipe, it was discovered that the infiltration is located in the area of the weld where cracks were found.

Fig. 1 Crack position and morphology of the sulfuric acid tube
To identify the cause of corrosion cracking and prevent the risk of recurrence, this article aims to analyze the chemical composition, metallographic microscope and scanning electron microscope of the defective stainless steel tube. By doing so, we can determine the root cause of the failure and suggest effective preventative measures.
1. Test method
(1) Chemical composition analysis
The ARL-4460 direct reading spectrometer is used to detect the chemical composition of the base metal and weld of stainless steel pipe, in order to determine whether they meet the requirements of the standard.
(2) Metallographic microscopic analysis
Cut a sample from the liquid penetration point as shown in Figure 1c. The sample must include the base metal, the weld and the heat affected zone. Then perform pre-grinding, grinding, fine grinding and polishing on the sample.
Then, use an OLYMPUS-GX51 metallographic microscope to observe any non-metallic inclusions present in the sample. Then, attack the sample with an aqueous solution of ferric chloride and hydrochloric acid. Finally, observe the structure of the sample under a metallographic microscope as shown in Figure 2.

Figure 2 metallographic sample
(3) SEM analysis
Using hydraulic pliers, tear the sample along the crack, then scan and observe the crack surface using the Hitachi S-3400 thermal field emission scanning electron microscope. Then perform energy spectrum analysis with an EDAX energy spectrometer.
2. Results and discussion
(1) Chemical composition analysis
Table 1 shows the chemical composition of the base metal and weld of a stainless steel pipe.
As shown in Table 1, the chemical composition of the stainless steel pipe purchased by the company is lower than the standard for both the base metal and the weld metal. However, the content of other elements meets the requirements of the standard.
Chromium (Cr) is the main corrosion-resistant element in stainless steel. When the Cr content is low, the corrosion resistance of stainless steel will decrease.
Table 1 Chemical Composition of Stainless Steel Pipe Materials (Mass Fraction (%)
| Element | W | Yes | Mn | P | s | Cr | No |
| GB/T4237—2015 | ≤0.07 | ≤0.75 | ≤2.00 | ≤0.045 | ≤0.030 | 5:50pm~7:50pm | 8.0~10.5 |
| Base metal | 0.07 | 0.39 | 0.99 | 0.033 | 0.011 | 5:36 p.m. | 10.14 |
| Weld bead | 0.07 | 0.40 | 1.00 | 0.030 | 0.013 | 16.85 | 10.03 |
(2) Metallographic microscopic analysis
Initially, the specimen was subjected to mechanical polishing and the distribution of non-metallic inclusions was observed under a microscope without chemical attack.
Upon observation, it was found that there were few non-metallic inclusions, but single, large-sized inclusions classified as Ds2 were present (see Fig. 3a).
The presence of non-metallic inclusions can disturb the continuity of the matrix, reduce its mechanical properties and increase its susceptibility to fractures.
Non-metallic inclusions can also reduce the thickness of the passive film (oxide film) formed on the surface of the stainless steel substrate, first leading to corrosion of the junction between the inclusions and the substrate. Subsequently, local corrosion at the interface can extend to the substrate, resulting in pitting corrosion.
Furthermore, the presence of non-metallic inclusions can promote grain boundary embrittlement and intergranular corrosion, thus reducing the corrosion resistance of the material.


Fig. 3 Microstructure of the sample fracture after corrosion
The polished sample was subjected to chemical attack and its structure was observed under a metallographic microscope.
Figure 3b represents the micrograph of the base metal of the sample. The structure is single-phase austenite (with twins), with no abnormalities found in the grain boundary. The average grain size of the metal is grade 7.
Figure 3c shows the microstructure of the fusion zone (left weld, right heat-affected zone). This zone has a normal structure, good fusion and no cracks, pores or other welding defects.
The metallographic microstructure is observed close to the crack (zone affected by welding heat), as shown in Figure 3d. Microcracks distributed along the grain boundary are clearly visible, with Cr network grain carbide forming a chromium-poor zone, as shown in Figure 4.
A chromium content (mass fraction) greater than 12% produces an obvious passivation effect, significantly improving the corrosion resistance of stainless steel. A chromium content of less than 12% destroys the passivation state, causing a drop in potential, and the passivation state remains in the crystal, forming a microgalvanic cell with a small anode (chromium-poor area in the grain boundary area) and a large cathode (matrix). This accelerates grain boundary corrosion.
The precipitation temperature of Cr 23 C 6 carbide is 450-850 ℃, which is the sensitization temperature range of intergranular corrosion of stainless steel, also known as the dangerous temperature range.
The above morphological characteristics show that there is sensitization in this area after welding, leading to intergranular corrosion in the weld heat-affected zone and reducing the intergranular corrosion resistance of the heat-affected zone of stainless steel. This is one of the reasons why stainless steel pipes crack.
(3) Fracture scan observation
Place the processed fracture sample into the scanning electron microscope to perform microscopic observation and analysis using secondary electron images.
As illustrated in Figure 4, it is evident that the fracture is irregular, with numerous corrosion products and cracks distributed in a dendritic pattern.
The cracks have secondary characteristics and penetrated the material matrix, indicative of stress corrosion cracking as the cause of failure in the 304 stainless steel tube.
Stainless steel has low thermal conductivity and welding generates residual stresses due to high temperatures.
Corrosion microcracks in stainless steel pipe accelerate under residual stress, leading to stress corrosion.

Fig. 4 SEM observation of fracture morphology
(4) Energy spectrum analysis
The energy spectrometer was used to analyze the corrosion products on the fracture surface of the stainless steel tube. Figure 5 presents the results of the power spectrum analysis.
From the diffraction peak spectrum, it is evident that the chlorine content is exceptionally high, indicating that the stainless steel pipe is exposed to a chlorine-containing corrosion environment.
The cracked steel pipes from the fertilizer factory are stored outdoors.
The plant site is situated in the coastal zone, just 1.1 km from the coast, which is a typical marine atmospheric environment.
During periods of high temperature and humidity, seawater evaporates in large quantities, producing salt fog that results in a high concentration of chloride ions in the air.
Water containing chloride ions is adsorbed on the outer wall of the stainless steel pipe, forming a corrosive medium that continuously corrodes the stainless steel pipe.
Austenitic stainless steel naturally forms a dense passivation film (oxide film) on its surface in a common atmospheric environment.
This passivation film isolates the atmosphere from direct contact with the stainless steel surface, providing excellent corrosion resistance and protection.
Even if the passive film is damaged, it can be regenerated and repaired in a timely manner.
However, chloride ions easily destroy the passivation film of austenitic stainless steel, leading to the formation of pits or pits on the surface and accelerating the corrosion of stainless steel.

3. Conclusion
Corrosion cracking of stainless steel pipes, in this case, cannot be attributed to a single factor. Instead, it is caused by the joint action of multiple factors.
(1) Non-metallic inclusions can damage the integrity of the passive film on the metal surface, reducing the corrosion resistance of stainless steel. Therefore, it is important to strictly control non-metallic inclusions below Level 1.5.
(2) The low Cr content in the base metal and weld metal reduces the compaction of the passive chromium film on the stainless steel surface. To improve the quality of steel pipes and welding materials, incoming components must be rigorously tested to ensure that the weld metal composition is not weaker than the base metal.
During the welding process, welding parameters must be strictly controlled and the welding heat input must be as small as possible to avoid sensitization, which can cause Cr to precipitate along the grain boundary and generate Cr 23 C 6 leading to intergranular corrosion of stainless steel.
(3) The chemical fertilizer factory is located in a marine atmospheric environment, where the high content of chloride ions in the air, suitable temperature and humidity accelerate corrosion. This causes the oxide film on the surface of stainless steel to be easily damaged, resulting in electrochemical corrosion.
Corrosion microcracks expand rapidly under the effect of residual stress, leading to stress corrosion cracking.
Therefore, it is necessary to strictly control the site's air environment and isolate the salt spray environment (e.g. by painting or adding a protective layer) to avoid damage caused by chloride ions.