Preface
35MnB steel is widely used in construction machinery tracked chassis components due to its excellent hardenability and superior performance in heat treatment processes.
Examples of such parts include roller wheel bodies, track chain rail links, tooth blocks, and other wear-resistant components.

1. Effect of main elements on 35MnB steel
Given the harsh operating conditions of its applications, the use of 35MnB steel requires that it be in a quenched and tempered state.
Hardenability and hardenability are crucial indicators for quenched and tempered steel, and their values are typically maintained by strictly controlling key elements such as Carbon (C), Silicon (Si), Manganese (Mn), Boron (B) and Chromium (Cr). , which have significant impacts on hardenability.
1.1 Effect of C content
The carbon content in 35MnB steel determines the hardness achievable after quenching. A higher carbon content leads to greater quenching hardness, but also increases the risk of cracking and reduces the plasticity and impact resistance of the steel.
For crucial components such as the track chassis, to minimize the effect of carbon content fluctuation on the surface hardness and quenching layer depth, it is necessary to establish requirements for the selection of carbon content. Generally, the upper and lower limits of carbon content are controlled within a range of 0.05%.
1.2 Effect of Si content
In addition to increasing strength and hardenability, the silicon in 35MnB steel also helps eliminate gases from the steel and stabilize it during steelmaking.
However, as the silicon content increases, the plasticity and toughness of the steel decrease, making it prone to forming a banded structure.
1.3 Effect of Mn content
Manganese (Mn), which is the main alloying element of 35MnB steel, improves the hardenability of the steel and reduces its critical cooling rate. Mn forms a solid solution with ferrite during heating, increasing the steel's strength. Mn is normally used when the depth of the hardened layer is greater than 4 mm. This is because it reduces the critical cooling rate, resulting in a more uniform quench hardness even when cooling conditions are not stable.
As shown in Figures 1 and 2, when the Mn content in steel is 1.10%, it greatly improves the strength of the steel with only a small decrease in plasticity and a slight improvement in toughness. However, if the Mn content exceeds this amount, the hardenability and strength will continue to improve, but the toughness will drop significantly.
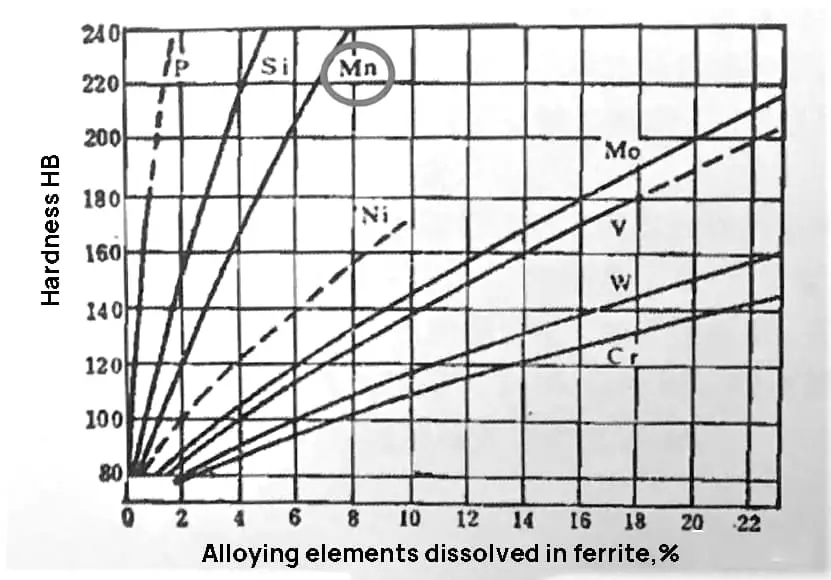
Fig. 1 Effect of alloying elements on solid solution strengthening
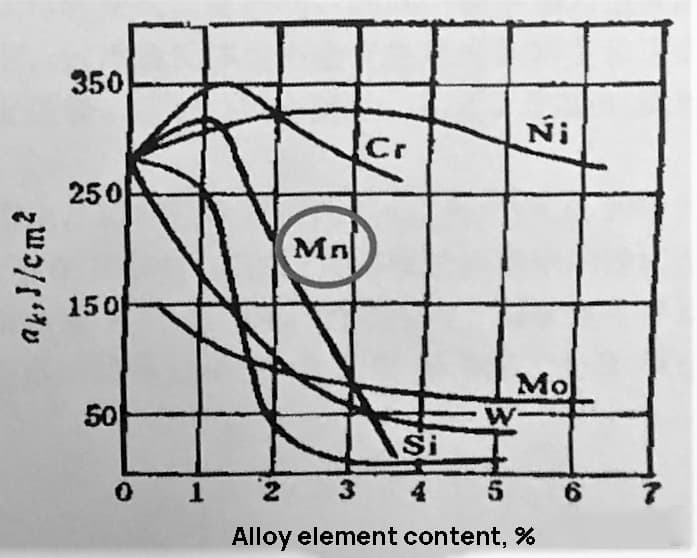
Fig. 2 Effect of alloying elements on ferrite impact energy
1.4 Effect of content B
In quenched and tempered high-strength steels, the addition of alloy element B can increase hardenability. This is achieved by dissolving a small amount of B in high-temperature austenite. During cooling, B will segregate at the austenite grain boundaries, inhibiting ferrite nucleation and thus improving hardenability, especially at low cooling rates.
However, B in steel is an active element that reacts easily with N to form stable BN, which is insoluble at quenching temperatures. This reduces the amount of effective B in the austenite solid solution and reduces its hardenability-enhancing effects.
To improve hardenability, it is necessary to add nitride-forming elements, control N elements, and maintain the amount of B solid solution in austenite. Furthermore, when the B content exceeds 30 ppm, the plasticity and strength of the material will decrease significantly.
Elements such as V, Ti, Al and B are strong nitride-forming elements in steel and form the nitrides VN, AlN, BN and TiN, respectively. When added to B-containing steel, the N in the steel will preferentially precipitate as TiN or Ti(C,N). This precipitation begins at temperatures above 1400°C, much higher than the initial precipitation temperature of BN. As the temperature decreases, the proportion of solid N in TiN increases, fixing N in the steel and preventing the formation of BN, thereby increasing the effective B content in austenite and improving hardenability.
To maximize the effective B content, it is important to control the TiN ratio in the steel, with an ideal value of 3.42. If the ratio is less than 3.42, the residual N content will increase and BN precipitation will occur, reducing the effective B content, hardenability and increasing brittleness. To avoid these effects, it is important to strictly control the residual N content in the steel.
1.5 Effect of Cr content
Cr is an element that greatly increases the hardenability of steel.
The addition of Cr to medium carbon chromium steel increases the incubation period of phase transformation, causing the isothermal transformation curve to shift to the right. This also causes pearlite transformation to occur at higher temperatures and bainite transformation to occur at lower temperatures.
As a result, when the right amount of chromium is added to steel, even with slow cooling during the quenching process, the undercooled austenite will not transform into pearlite or bainite before reaching the martensite transformation temperature, significantly improving hardenability. of steel.
However, Cr also significantly worsens the temper brittleness of nickel and manganese steels. Therefore, the Cr content in 35MnB steel is carefully regulated.
Studies on the effect of Cr trace on the hardenability of 35MnB tracked binding steel indicate that even small changes in Cr content (Cr ≤ 0.20%) can significantly impact hardenability, especially when the Cr content exceeds 0.10 %. This significantly improves the hardness of the steel, especially at points away from the water-cooled end.
The figure below shows that quenching hardness can increase by an average of 2 to 3 HRC in the range of 1.5 to 20.0 m from the water-cooled end. When the distance from the water-cooled end is greater than 20.0 m, the hardness increases further, by about 6 HRC.
Furthermore, the hardenable round bar diameter of 35Mnb steel containing Cr0.18% is about 20mm larger than that of steel containing Cr0.02%.
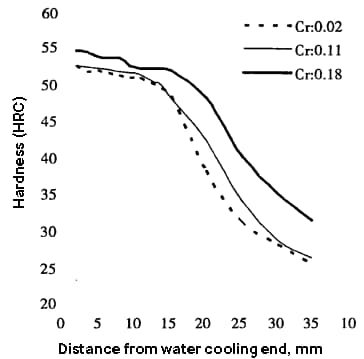
Fig. 3 Effect of Cr content on hardenability
Since Cr has the ability to form carbides, it requires an increase in heating temperature and a prolonged heating time, which is not ideal for induction hardening.
2. Tin damage to 35MnB steel
During the steel manufacturing process, tin's high melting point causes it to precipitate in the liquid phase before smelting and solidifying. This results in the formation of tin particles in the liquid, which are typically 2 to 10 μm in size.
These particles have a square, diamond or triangular shape (different from BN, as shown in Fig. 6) and have extremely high hardness (greater than 1000V).
As shown in Figure 4 and Figure 5, these particles cannot be changed through any processing method and cannot be dissolved through high-temperature solid solution. Furthermore, they lead to a large dispersion of impact energy.
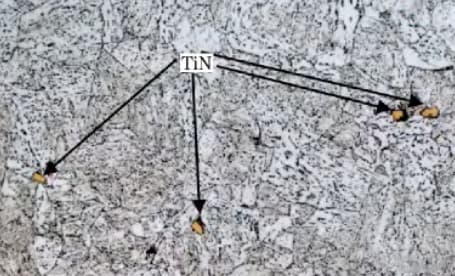
Fig. 4 observation under tin optical microscope
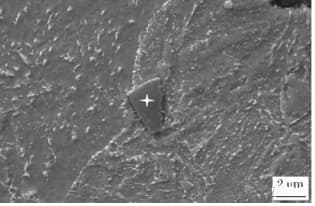
Fig. 5 Observation of tin under electron microscope
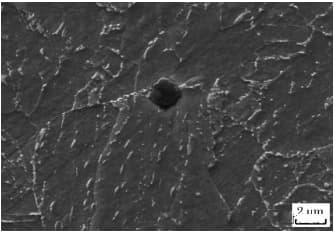
Fig. 6 observation of BN under electron microscope
Fig. 7 is a solubility product curve in liquid iron at 1400°C, 1450°C and 1500°C;
As shown in the figure, when the temperature of molten steel at the beginning of solidification is 1500°C, the presence of 80 ppm N and more than 0.043% Ti in the steel will result in liquid and tin precipitation. Similarly, when the N content in steel is 40ppm and the Ti content exceeds 0.086%, there will be liquid and tin precipitation.
When the final solidification temperature of dendritic molten steel is 1400°C, the presence of 80 ppm N and more than 0.012% Ti will result in liquid and tin precipitation. Furthermore, if the N content in steel is 40ppm and the Ti content exceeds 0.024%, there will be liquid and tin precipitation.
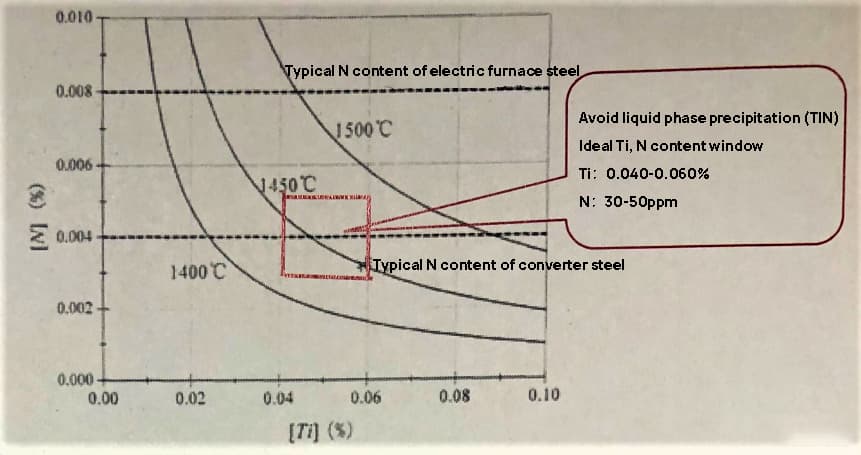
Fig. 7 Tin solubility product curve
To prevent the occurrence of tin liquidus, it is crucial to properly adjust the Ti and N content in the steel. This will suppress tin liquidus precipitation during solidification and increase the cooling rate during casting to reduce precipitation in the last solidified molten steel. By accelerating the rate of cooling, there will not be enough time for precipitation to occur dynamically.
Calculations of the tin solubility product in liquid iron show that the final solidification temperature during smelting and casting is approximately 1495°C, with an equilibrium tin solubility product of 0.00302.
If the N content is controlled at 80 ppm, the maximum amount of tin that can be dissolved in liquid iron at the final solidification temperature is 0.0413%. To avoid liquid tin precipitation, the chemical composition must have a Ti content ≤ 0.0413%.
If the nitrogen content is controlled at 60 ppm, the maximum T content that can be dissolved in liquid iron at the final solidification temperature is 0.05%. To avoid the production of tin liquidus, the designed Ti content of the steel's chemical composition must be ≤ 0.05%.
To increase the effective B content in 35MnB steel, the N content in the steel must be reduced to less than 60 ppm.
If the liquid phase precipitation of tin exceeds 6 μm, it can significantly reduce the material's service life and impact resistance. If it exceeds 6 μm, it should be judged as Al 2 Ó 3 brittle inclusion.
Inclusions such as tin, Al 2 Ó 3 MgO · Al 2 Ó 3 and Cao · Al 2 Ó 3 , which are hard and brittle, do not show plasticity under deformation temperature. They easily separate from the body structure during deformation, impairing its continuity. In severe cases, cracks or cavities may appear at the edge of the undeformed inclusion.
In service, alternating stresses can easily cause stress concentration, becoming a source of metal fatigue.
3. Summary
Good control of material composition is essential to ensure material performance. The recommended composition (in weight percentage) for 35MnB material during melting is as follows:
| Note | 35MnB |
| W | 0.32-0.36 |
| Yes | 0.15-0.35 |
| Mn | 1.1-1.4 |
| P | ≤0.025 |
| s | 0.025 |
| Cr | 0.15-0.25 |
| No | 0.2 |
| Ass | 0.25 |
| B | 0.0005-0.003 |
| Al | 0.015-0.045 |
| You | ≤0.05 |
| Mo | ≤0.05 |
| 【H】 | ≤2ppm |
| 【O】 | ≤18ppm |
| 【N】 | ≤60ppm |

























































