-

Propriedades Mecânicas dos Metais: Entendendo a Resistência e Durabilidade
Frequentemente, os materiais estão sujeitos a uma força externa quando são usados. Engenheiros mecânicos calculam essas forças, e cientistas de materiais determinam como os materiais se deformam ou...
-

Entendendo os diferentes tipos de Tubos de Aço: ERW, DOM e Sem Costura
Este mês, examinamos as diferenças entre ERW, DOM e tubos sem costura. Tubos ERW (Electric Resistance Welded) ERW se refere a um processo de soldagem que envolve soldagem por pontos e por costura, ...
-

Como verificar a dureza do metal sem uma máquina Rockwell
Por muito tempo, pensei que a única maneira de verificar corretamente a dureza do metal era com uma máquina de teste Rockwell adequada. Felizmente, aprendi algumas outras técnicas simples e eficaze...
-

Curso com Planilhas Automáticas para Engenharia Civil
Planilhas Automáticas para Engenharia Civil: Cálculo e Dimensionamento Simplificados
Na engenharia civil, precisão e eficiência são vitais para o sucesso de qualquer projeto. No entanto, o process...
-

Curso de Power BI para Engenharia Civil
Power BI na Engenharia Civil: Da Introdução às Aplicações Avançadas
No cenário competitivo da engenharia civil e da construção, a capacidade de gerenciar e analisar dados se tornou uma habilidade ...
-

Cursos de Soldagem: Programa Completo para Iniciantes e Profissionais
Qualificação Profissional em Soldagem: Cursos Estratégicos para Atuar com Sucesso na Indústria
A soldagem é uma das habilidades mais críticas e requisitadas na indústria, especialmente em setores...
-

Como prolongar a Vida Útil do Bico de Contato na Solda MIG
A solda MIG (Metal Inert Gas) é uma técnica amplamente utilizada na indústria e construção devido à sua eficiência, versatilidade e qualidade dos resultados. No entanto, um componente crucial neste...
-

Como manter o Arco Elétrico Estável durante a Soldagem
A soldagem é uma técnica essencial em diversas indústrias, desde a construção civil até a fabricação de automóveis. No entanto, um dos desafios mais comuns enfrentados pelos soldadores é a manutenç...
-

Como Eliminar Respingos Excessivos na Soldagem MIG
A soldagem MIG (Metal Inert Gas) é uma técnica amplamente utilizada na indústria e construção, conhecida por sua eficiência e versatilidade. No entanto, um dos desafios comuns enfrentados pelos pro...
-

Como escolher o arame correto para solda MIG
A escolha do arame correto para solda MIG é fundamental para obter resultados de alta qualidade e eficiência no processo de soldagem. Cada tipo de arame possui características específicas que afeta...
-

Soldagem de Tubulações em Campo: Preparação e Sequenciamento
A soldagem de tubulações em campo é um desafio único que requer habilidades especializadas e planejamento cuidadoso. Diferente da soldagem em ambiente controlado de oficina, a soldagem em campo enf...
-

Soldagem em Peças Pintadas: Evitando Problemas e Garantindo Segurança
A soldagem é uma técnica fundamental em diversos setores industriais, desde a fabricação de automóveis até a construção civil. No entanto, quando se trata de soldar peças que já foram pintadas, é e...
-

Escovas de Aço na Soldagem: Preparação e Acabamento Perfeitos
A soldagem é uma técnica fundamental em diversas indústrias, desde a construção civil até a fabricação de maquinário pesado. No entanto, para obter resultados de alta qualidade, é essencial prepara...
-

Entendendo o Processo de Brasagem: Quando e Como Utilizá-lo
A brasagem é uma técnica de união de metais que se diferencia da solda tradicional por fusão. Enquanto a solda envolve o derretimento e a mistura dos metais, a brasagem utiliza uma liga de metal de...
-

Importância da Limpeza Pós-Soldagem em Aço Inox e Alumínio
A soldagem é uma técnica fundamental em diversos setores industriais, desde a construção civil até a fabricação de equipamentos. No entanto, após o processo de soldagem, é essencial realizar uma li...
-

Identificando e Corrigindo a Falta de Penetração na Solda
A integridade estrutural de uma junta soldada depende crucialmente da qualidade da penetração da solda. Quando a penetração é insuficiente, a resistência da união fica comprometida, podendo levar a...
-

Processo de Soldagem por Resistência Elétrica: Entendendo os Detalhes Técnicos
A soldagem por resistência elétrica, também conhecida como solda por ponto ou spot weld, é uma técnica amplamente utilizada na indústria, especialmente na fabricação de automóveis e estruturas metá...
-

Soldagem em Aço Inoxidável: Evitando os Erros Mais Comuns
A soldagem de aço inoxidável é uma tarefa delicada que requer atenção aos mínimos detalhes. Diferente da soldagem de aços carbono, o processo de união de peças em aço inoxidável envolve desafios es...
-

Importância do Controle da Velocidade de Avanço na Soldagem
A soldagem é uma técnica fundamental em diversas indústrias, desde a construção civil até a fabricação de automóveis. No entanto, para obter resultados de alta qualidade, é essencial dominar divers...
-

Soldagem de Titânio: Superando Desafios e Impulsionando a Inovação
O titânio é um material fascinante, conhecido por sua resistência, leveza e biocompatibilidade. No entanto, a soldagem desse metal nobre apresenta desafios únicos que exigem técnicas especializadas...
-

Como usar gabaritos e fixações para garantir soldagens precisas
A precisão é fundamental quando se trata de soldagem. Peças mal alinhadas ou deslocadas podem resultar em juntas defeituosas, reduzindo a integridade estrutural e a aparência final do trabalho. Fel...
-

Técnicas Essenciais de Ponteamento para Montagem de Estruturas Metálicas
A montagem de estruturas metálicas é uma etapa crucial no processo de construção, exigindo técnicas precisas e cuidadosas para garantir a estabilidade e o alinhamento adequado das peças. Uma das té...
-

Poça de Fusão: Técnicas Essenciais para Soldagem TIG Perfeita
A soldagem TIG (Tungsten Inert Gas) é uma técnica versátil e precisa, amplamente utilizada na indústria e construção. No entanto, manter a poça de fusão sob controle pode ser um desafio, especialme...
-

Soldagem a Plasma: Tecnologia para a Indústria e Construção
A soldagem a plasma, também conhecida como PAW (Plasma Arc Welding), é uma técnica de soldagem avançada que vem revolucionando a indústria e a construção. Essa tecnologia oferece uma solução eficie...
-

Importância do Martelo de Escória na Soldagem com Eletrodo Revestido
A soldagem com eletrodo revestido é uma técnica amplamente utilizada na indústria e construção civil, sendo essencial para a união de metais e a fabricação de estruturas robustas. No entanto, o pro...
-

Como fazer uma Solda Filetada com Qualidade Profissional
A solda filetada, também conhecida como solda em ângulo, é uma técnica amplamente utilizada na indústria e na construção civil para unir peças metálicas em ângulo. Essa técnica é essencial para a f...
-

Entendendo os Tipos de Soldagem: Manual, Semiautomática e Automática
A soldagem é uma técnica fundamental em diversos setores industriais, desde a fabricação de automóveis até a construção civil. No entanto, nem todas as técnicas de soldagem são iguais. Existem três...
-

Entendendo a Escorificação na Soldagem: Causas, Impactos e Soluções
A soldagem é uma técnica fundamental em diversos setores industriais, desde a construção civil até a fabricação de máquinas e equipamentos. No entanto, durante o processo de soldagem, um fenômeno i...
-

Diferença entre solda TIG DC e TIG AC: quando usar cada uma
A solda TIG (Tungsten Inert Gas) é uma técnica amplamente utilizada na indústria e construção, conhecida por sua precisão, qualidade e versatilidade. No entanto, existem duas variantes principais d...
-

Soldagem em Aço Carbono Espesso: Desafios e Soluções
A soldagem de aço carbono espesso é um desafio constante para profissionais da indústria e construção. Essas peças exigem cuidados especiais durante o processo de soldagem, a fim de garantir a inte...
-

Como a Soldagem Robotizada Transforma a Indústria
A evolução tecnológica tem impulsionado transformações significativas em diversos setores industriais, e a soldagem robotizada é um exemplo claro dessa realidade. Essa técnica avançada vem se conso...
-

Soldar Aço 1020 corretamente: Evite trincas e Garanta Qualidade
O aço SAE AISI 1020 é um dos materiais mais utilizados na indústria e construção civil devido à sua versatilidade e custo-benefício. Esse aço carbono de baixa liga é amplamente empregado em estrutu...
-

Como Soldar Aço Inox com TIG Sem Contaminação: Um Guia Passo a Passo
A soldagem de aço inoxidável é uma tarefa delicada que requer atenção especial para evitar contaminação e obter um acabamento limpo e profissional. A técnica de soldagem TIG (Tungsten Inert Gas) é ...
-

Como identificar e utilizar o Eletrodo 6013
O eletrodo 6013 é um dos tipos mais comuns e versáteis de eletrodos de soldagem utilizados na indústria e construção civil. Sua popularidade se deve às suas características únicas, que o tornam ide...
-

Como fazer uma Solda no Eletrodo Revestido
A solda com eletrodo revestido é uma técnica amplamente utilizada na indústria e construção civil devido à sua versatilidade e facilidade de aplicação. Neste artigo, vamos explorar detalhadamente o...
-

Garantia de Qualidade em Soldas: Os Principais Testes Não Destrutivos
A integridade e a qualidade das soldas são fundamentais em diversos setores industriais, desde a construção civil até a fabricação de equipamentos de alta tecnologia. Para assegurar que as juntas s...
-

Como ajustar Corretamente a Amperagem na Solda com Eletrodo Revestido
A solda com eletrodo revestido é uma técnica amplamente utilizada na indústria e construção, sendo essencial para a união de peças metálicas. Um dos fatores críticos neste processo é o ajuste preci...
-

Equipamentos de Proteção Individual (EPIs) obrigatórios para Soldadores
A segurança é um fator primordial no ambiente de trabalho, especialmente para profissionais que lidam com atividades de alto risco, como a soldagem. Os Equipamentos de Proteção Individual (EPIs) sã...
-

Diferença entre Solda por Resistência e Solda por Fusão: Aplicações Práticas
A escolha do processo de soldagem correto é crucial para garantir a integridade e a eficiência de diversas aplicações industriais, desde a fabricação de automóveis até a construção de aeronaves. Ne...
-

A Soldagem Orbital: Precisão e Eficiência
A soldagem orbital é uma técnica revolucionária que está transformando a maneira como as indústrias abordam a união de materiais. Essa abordagem automatizada e precisa tem se destacado em diversos ...
-

Como Regular sua Inversora MIG para Chapas Finas
A solda MIG (Metal Inert Gas) é uma técnica amplamente utilizada na indústria e construção, conhecida por sua versatilidade e eficiência. No entanto, quando se trata de trabalhar com chapas finas, ...
-

5 Erros Comuns na Soldagem com Alumínio
A soldagem de alumínio é uma técnica desafiadora que requer atenção aos detalhes e conhecimento especializado. Infelizmente, muitos profissionais cometem erros comuns que podem comprometer a qualid...
-

Como Prevenir Trincas a Quente e a Frio em Soldas
A soldagem é uma técnica fundamental na indústria e construção, permitindo a união de peças metálicas de forma eficiente e resistente. No entanto, um desafio comum enfrentado pelos profissionais é ...
-

Soldagem com Arame Tubular: Praticidade, Penetração e Produtividade
A soldagem com arame tubular, também conhecida como FCAW (Flux-Cored Arc Welding), é um processo de soldagem amplamente utilizado na indústria e na construção civil. Essa técnica combina a praticid...
-

Diferença entre Solda Forte e Solda Branda
A escolha entre solda forte e solda branda é uma decisão crucial para muitos profissionais da indústria e construção. Cada uma dessas técnicas de união possui características únicas que as tornam a...
-

Soldagem por Fricção: Junção de Metais Leves
A indústria moderna enfrenta constantes desafios na busca por soluções de fabricação cada vez mais eficientes e sustentáveis. Nesse cenário, a técnica de soldagem por fricção (FSW - Friction Stir W...
-

Soldagem em Campo: Desafios e Soluções para Ambientes Adversos
A soldagem é uma técnica essencial em diversos setores industriais, desde a construção civil até a fabricação de equipamentos. No entanto, quando a soldagem precisa ser realizada fora do ambiente c...
-

Soldagem por Arco Submerso: Eficiência e Versatilidade na Indústria
A soldagem por arco submerso (SAW) é uma técnica amplamente utilizada na indústria, conhecida por sua alta taxa de deposição e capacidade de soldar chapas grossas e estruturas pesadas. Este process...
-

A Solda de Tampão: Unindo Chapas com Eficiência e Resistência
A solda de tampão, também conhecida como solda de pino ou solda de ponto, é uma técnica amplamente utilizada na indústria e na construção civil para unir chapas sobrepostas de maneira rápida, efici...
-

Como obter um Acabamento Perfeito na Solda com Esmerilhamento Correto
Na indústria e construção, o acabamento superficial da solda é um fator crucial para a aparência final e a integridade estrutural de um projeto. Um acabamento mal feito pode comprometer a resistênc...
-

Como Soldar Aço Galvanizado sem comprometer a Proteção Anticorrosiva
A solda é uma técnica essencial na indústria e construção, permitindo a união de peças metálicas de forma segura e eficiente. No entanto, quando se trata de soldar aço galvanizado, existem desafios...
-

A Soldagem por Feixe de Elétrons: Técnica Avançada para Unir Metais com Precisão
A soldagem por feixe de elétrons (EBW) é uma técnica avançada de união de metais que utiliza um feixe de elétrons acelerados em uma câmara de vácuo para fundir e soldar materiais com precisão milim...
-

Soldagem em Tubulações: Desafios e Técnicas Essenciais
A soldagem em tubulações é uma tarefa crítica na indústria e construção, exigindo habilidades especializadas e técnicas avançadas. Neste artigo, exploraremos os desafios técnicos envolvidos nesse t...
-

Como o ângulo da tocha afeta a solda MIG
A solda MIG (Soldagem por Gás de Metal) é uma técnica amplamente utilizada na indústria e construção, conhecida por sua eficiência e versatilidade. Um dos fatores-chave que influenciam diretamente ...
-

Soldagem em Alumínio: Principais cuidados e Técnicas Recomendadas
O alumínio é um material amplamente utilizado na indústria e na construção civil devido às suas propriedades únicas, como leveza, resistência à corrosão e alta condutividade elétrica e térmica. No ...
-

Como lidar com Rachaduras na Solda ao Resfriar o Cordão
A soldagem é uma técnica fundamental na indústria e construção, permitindo a união de peças metálicas de forma eficiente e resistente. No entanto, um desafio comum que os profissionais enfrentam é ...
-

Pisos de Madeira vs. Pisos de Cerâmica: Qual é a melhor opção para sua casa?
Ao escolher o piso ideal para sua casa, você provavelmente se depara com a decisão entre pisos de madeira e pisos de cerâmica. Ambos os tipos de piso têm suas próprias vantagens e desvantagens, e a...
-

Paredes de Drywall vs. Paredes de Gesso: Qual é a melhor opção para sua construção?
Ao planejar uma construção ou reforma, uma das decisões mais importantes a ser tomada é a escolha do material para as paredes. Duas opções populares são as paredes de drywall e as paredes de gesso....
-

Pisos de Granito vs. Pisos de Porcelanato: Qual é a melhor opção para sua casa?
Ao escolher o piso ideal para sua casa, é importante considerar os prós e contras de diferentes opções. Neste artigo, vamos explorar as diferenças entre os pisos de granito e os pisos de porcelanat...
-

Construção Sustentável vs. Construção Tradicional: Qual é a melhor opção?
A indústria da construção civil tem sido um dos principais motores da economia global, mas também um dos maiores contribuintes para os desafios ambientais que enfrentamos atualmente. Diante dessa r...
-
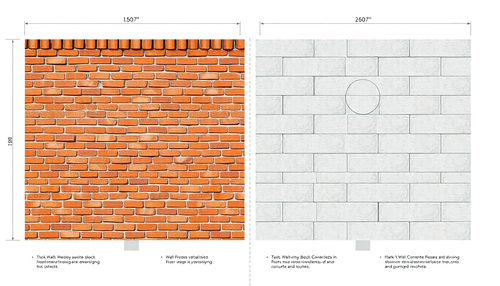
Paredes de Tijolos vs. Paredes de Blocos de Concreto: Qual a Melhor Opção para sua Construção?
Ao planejar uma construção, uma das decisões mais importantes a ser tomada é a escolha do material para as paredes. Duas opções populares são as paredes de tijolos e as paredes de blocos de concret...
-

Telhados de Zinco vs. Telhados de Cerâmica: Qual é a melhor opção para sua Casa?
Ao escolher o material ideal para o telhado de sua casa, é importante considerar diversos fatores, como durabilidade, custo, desempenho térmico e estética. Neste artigo, vamos explorar as principai...
-

Hidrocerâmica vs. Isolamento Tradicional: Qual a melhor opção para sua Construção?
A escolha do sistema de isolamento térmico é uma decisão crucial para qualquer projeto de construção, seja residencial ou comercial. Neste artigo, vamos explorar as diferenças entre a hidrocerâmica...
-

Janelas de Vidro vs. Janelas de PVC: Qual a Melhor Opção para Sua Casa?
Ao escolher as janelas certas para sua casa, você se depara com uma decisão importante: vidro ou PVC? Ambos os materiais têm suas próprias vantagens e desvantagens, e a escolha certa dependerá das ...
-
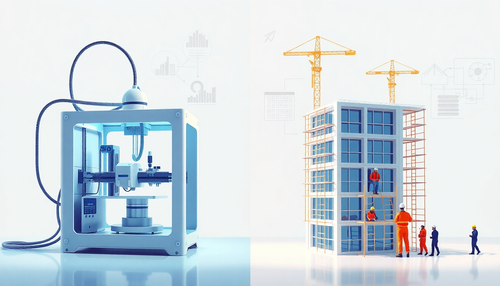
Impressão 3D vs. Construção Tradicional: Explorando as Vantagens e Desvantagens de Cada Abordagem
A indústria da construção civil está passando por uma transformação significativa, com a introdução de novas tecnologias que desafiam os métodos tradicionais. Entre essas inovações, a impressão 3D ...
-

Aerogel vs. Isolamento Tradicional: Qual é a melhor opção para sua construção?
Quando se trata de isolamento térmico em construções, duas opções se destacam: o aerogel e o isolamento tradicional. Cada uma dessas soluções possui suas próprias vantagens e desvantagens, e a esco...
-

Grafeno vs. Fibra de Carbono: Qual Material é melhor para sua Aplicação?
A escolha entre grafeno e fibra de carbono é uma decisão importante para muitas indústrias, desde a aeroespacial até a construção civil. Ambos os materiais oferecem características únicas e vantage...
-

Espuma de Alumínio vs. Isolamento de Lã de Vidro: Qual é a melhor opção para sua Construção?
Ao escolher o material de isolamento ideal para sua construção, é importante considerar os prós e contras de cada opção. Neste artigo, vamos explorar as diferenças entre a espuma de alumínio e o is...
-

Painéis Solares vs. Telhados Tradicionais: Qual a melhor opção para sua casa?
Ao considerar as opções para sua casa, você provavelmente se depara com a escolha entre painéis solares e telhados tradicionais. Cada uma dessas soluções tem suas próprias vantagens e desvantagens,...
-

Pisos de Vinil vs. Pisos de Porcelanato: Qual é a melhor opção para sua casa?
Ao escolher o piso ideal para sua casa, é importante considerar fatores como durabilidade, facilidade de instalação e resistência à umidade. Neste artigo, vamos explorar as principais diferenças en...
-
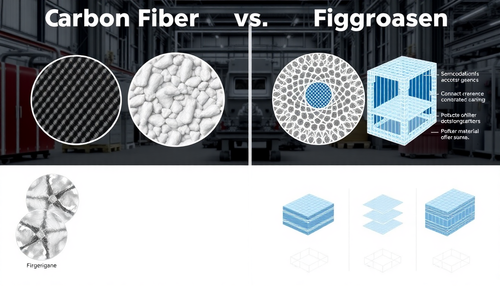
Fibras de Carbono vs. Fibras de Vidro: Qual a melhor escolha para sua Indústria?
A escolha entre fibras de carbono e fibras de vidro é uma decisão importante para muitas indústrias, desde a construção civil até a aeroespacial. Ambos os materiais têm suas próprias vantagens e de...
-

Concreto Reciclado vs. Concreto Tradicional: Qual é a melhor opção para a Construção Sustentável?
A construção civil é um setor fundamental para o desenvolvimento econômico e social de um país, mas também é um dos maiores consumidores de recursos naturais e emissores de carbono. Nesse contexto,...
-

Grafeno vs. Nanopartículas de TiO2: Qual é a melhor opção para sua indústria?
A indústria está constantemente em busca de soluções inovadoras que possam melhorar a eficiência, reduzir custos e aumentar a sustentabilidade. Duas tecnologias que têm ganhado destaque nesse cenár...
-

BIM vs. Projetos Tradicionais: Qual a melhor opção para a sua Indústria?
A indústria da construção está em constante evolução, e duas abordagens se destacam: o BIM (Building Information Modeling) e os projetos tradicionais. Cada uma dessas metodologias possui suas própr...
-

Construção Modular vs. Construção Pré-Fabricada: Qual é a melhor opção para sua Obra?
A indústria da construção civil está em constante evolução, e duas abordagens têm se destacado: a construção modular e a construção pré-fabricada. Ambas oferecem vantagens e desvantagens, e a escol...
-
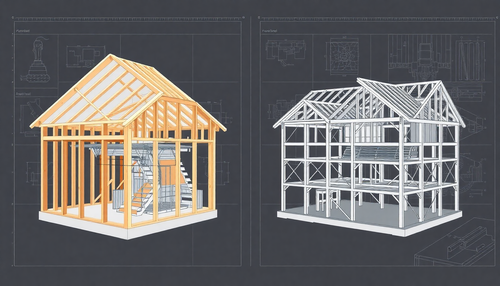
Light Steel Framing vs. Estruturas de Aço Tradicionais: Qual a melhor opção para sua Construção?
A escolha entre Light Steel Framing e Estruturas de Aço Tradicionais é uma decisão importante para qualquer projeto de construção. Ambas as opções têm suas próprias vantagens e desvantagens, e é es...
-

Fundações Profundas vs. Fundações Rasas: Melhor opção na Construção
A escolha entre fundações profundas e rasas é uma decisão crucial no mundo da construção civil. Cada uma dessas opções apresenta vantagens e desvantagens específicas, e a seleção da mais adequada d...
-

Concreto Convencional vs. Concreto de Alta Resistência (CAR): Entendendo as Diferenças e Escolhendo a Melhor Opção
Na indústria da construção, a escolha do tipo de concreto a ser utilizado é uma decisão crucial que pode impactar significativamente o desempenho, a durabilidade e o custo de uma obra. Neste artigo...
-

Concreto Convencional vs. Concreto com Fibra de Aço: Qual é a Melhor Opção para sua Construção?
Quando se trata de construção, a escolha do tipo de concreto a ser utilizado é uma decisão crucial que pode impactar significativamente o desempenho e a durabilidade de uma obra. Neste artigo, expl...
-

Concreto Autocicatrizante vs. Concreto Convencional: Qual é a melhor opção para sua Construção?
A escolha entre concreto autocicatrizante e concreto convencional é uma decisão importante para qualquer projeto de construção. Ambos os tipos de concreto têm suas próprias vantagens e desvantagens...
-

Concreto Convencional vs. Concreto Autoadensável: Qual é a melhor opção para sua obra?
O mundo da construção civil está em constante evolução, e uma das principais inovações neste setor é o concreto autoadensável. Embora o concreto convencional ainda seja amplamente utilizado, o conc...
-

Concreto Leve vs. Concreto Pesado: Escolhendo a solução ideal para Construção
Ao planejar uma construção, a escolha entre concreto leve e concreto pesado é uma decisão crucial que pode impactar significativamente o projeto. Cada tipo de concreto possui suas próprias caracter...
-

Concreto com Fibra de Polipropileno vs. Concreto Convencional: Qual é a melhor opção para sua Construção?
A escolha do tipo de concreto a ser utilizado em uma construção é uma decisão crucial que pode impactar significativamente a qualidade, durabilidade e eficiência do projeto. Neste artigo, vamos exp...
-

Concreto Pré-Moldado vs. Concreto Usinado: Qual é a melhor opção para Construção?
A escolha entre concreto pré-moldado e concreto usinado é uma decisão crucial para qualquer projeto de construção. Ambas as opções têm suas próprias vantagens e desvantagens, e a seleção da solução...
-

Concreto Reforçado com Fibras vs. Concreto Armado: Qual é a melhor opção para sua construção?
A escolha entre concreto reforçado com fibras e concreto armado é uma decisão importante para qualquer projeto de construção. Ambos os materiais têm suas próprias vantagens e desvantagens, e a sele...
-

Bioconcreto vs. Concreto Convencional: Qual a Melhor Opção para Sua Construção?
A indústria da construção civil está constantemente em busca de soluções mais sustentáveis e duráveis para seus projetos. Nesse contexto, o bioconcreto surge como uma alternativa promissora ao conc...
-

Argamassa Polimérica: Eficiência e Sustentabilidade na Construção
A construção civil está passando por uma transformação significativa, com a adoção de soluções inovadoras que visam aumentar a eficiência e a sustentabilidade dos processos. Uma dessas inovações é ...
-

Pavimentação Sustentável com Pisos Intertravados de Concreto
Os pisos intertravados de concreto têm se destacado como uma solução versátil e sustentável para a pavimentação de calçadas, praças e estacionamentos. Essa tecnologia modular oferece inúmeras vanta...
-

Como Implementar um Sistema de Captação e Reuso de Água
A escassez de água é um desafio global cada vez mais urgente. Com a crescente demanda por recursos hídricos e os impactos das mudanças climáticas, é essencial que adotemos soluções sustentáveis par...
-
Blocos de Concreto Ecológico: Uma Solução Sustentável para a Construção Civil
A construção civil é um setor fundamental para o desenvolvimento de uma sociedade, mas também é responsável por uma parcela significativa do impacto ambiental. No entanto, uma inovação crescente ne...
-

Concreto que se Cura Sozinho: A Revolução da Construção Sustentável
A construção civil é um setor fundamental para o desenvolvimento de qualquer sociedade, mas também enfrenta desafios significativos em termos de sustentabilidade e durabilidade das estruturas. No e...
-

Revolução da Construção: Impressão 3D de Casas e Edifícios
A construção civil está passando por uma transformação revolucionária com a adoção da tecnologia de impressão 3D. Essa inovadora abordagem está redefinindo a maneira como construímos casas e edifíc...
-

Futuro da Construção: Concreto Translúcido Revoluciona a Indústria
A indústria da construção está constantemente em busca de inovações que possam melhorar a eficiência, a sustentabilidade e a estética das estruturas. Uma dessas inovações que tem ganhado destaque n...
-

Cimento Auto-Adensável: Revolucionando a Indústria da Construção
A indústria da construção civil está em constante evolução, e uma das inovações que tem se destacado é o cimento auto-adensável (CAA). Essa tecnologia revolucionária está transformando a maneira co...
-
Blocos de Concreto com Isolamento Acústico: Soluções Eficientes para Ambientes Silenciosos
Os desafios acústicos em ambientes como hospitais, escolas e escritórios são uma preocupação constante para arquitetos, engenheiros e gestores de instalações. O ruído excessivo pode afetar negativa...
-

Fundações Resilientes: O Poder das Microestacas
As construções modernas enfrentam desafios cada vez mais complexos quando se trata de fundações. Terrenos instáveis, restrições de espaço e a necessidade de preservar estruturas existentes são apen...
-

Concreto Geopolimérico: Uma Alternativa Sustentável para a Construção Civil
O setor da construção civil enfrenta um desafio cada vez mais urgente: encontrar soluções sustentáveis que reduzam o impacto ambiental das obras e infraestruturas. Nesse contexto, o concreto geopol...
-

Drones: A Revolução na Inspeção e Monitoramento de Obras
A indústria da construção civil está passando por uma transformação significativa com a adoção de novas tecnologias, e uma das mais impactantes é o uso de drones para inspeção e monitoramento de ob...
-
Revolução do Concreto de Alto Desempenho em Estruturas Submersas
O mundo da construção está passando por uma transformação significativa, com o crescente uso de concreto de alto desempenho (HPC) em projetos de infraestrutura submersa. Essa tecnologia avançada es...
-

A Revolução da Escavação Robótica na Construção de Túneis
A construção de túneis sempre foi um desafio complexo e arriscado, exigindo técnicas e equipamentos especializados para lidar com as condições do solo e do subsolo. No entanto, nos últimos anos, a ...
-

Construção com Materiais Sustentáveis: Madeiras Laminadas e Painéis de Fibra de Coco
A construção com materiais sustentáveis tem sido uma prioridade crescente na indústria da construção civil, com materiais como madeira laminada e painéis de fibra de coco sendo usados para substitu...
-

Vidros Inteligentes: Transformando Edifícios e Lares
Os vidros inteligentes estão revolucionando a indústria da construção e da arquitetura, oferecendo soluções inovadoras para tornar os edifícios e residências mais eficientes, sustentáveis e adaptáv...
-

Pavimentos Permeáveis: Solução Sustentável para Áreas Urbanas
Os pavimentos permeáveis são uma inovação importante no gerenciamento de águas pluviais nas áreas urbanas. Eles permitem que a água da chuva passe por uma camada de material, como concreto ou asfal...
-

Reformas Rápidas e Eficientes com Sistemas de Construção a Seco
A construção civil está passando por uma transformação significativa, com a adoção cada vez mais frequente de sistemas de construção a seco. Essa tecnologia, que utiliza painéis de gesso acartonado...
-

Pintura Térmica: Solução Eficiente para Reduzir o Calor em Edificações
A busca por soluções sustentáveis e eficientes para melhorar o desempenho térmico de edifícios tem sido uma preocupação crescente entre arquitetos, engenheiros e proprietários de imóveis. Nesse con...
-

Concreto Autorreparável: Como as Bactérias estão Transformando a Construção Civil
O setor da construção civil enfrenta constantemente desafios relacionados à durabilidade e manutenção das estruturas. As fissuras e rachaduras são problemas comuns que podem comprometer a segurança...













































































































